Chapter: Basic & Clinical Pharmacology : Thyroid &Antithyroid Drugs
Thioamides
THIOAMIDES
The thioamides
methimazole and propylthiouracil are major drugs for treatment of
thyrotoxicosis. In the United Kingdom, carbimazole, which is converted to
methimazole in vivo, is widely used. Methimazole is about ten times more potent
than propylth-iouracil and is the drug of choice in adults and children. Due to
a black box warning about severe hepatitis, propylthiouracil should be reserved
for use during the first trimester of pregnancy, in thy-roid storm, and in
those experiencing adverse reactions to methi-mazole (other than
agranulocytosis or hepatitis). The chemical structures of these compounds are
shown in Figure 38–5. The thiocarbamide group is essential for antithyroid
activity.
Pharmacokinetics
Methimazole is
completely absorbed but at variable rates. It is readily accumulated by the
thyroid gland and has a volume of distribution similar to that of
propylthiouracil. Excretion is slower than with propylthiouracil; 65–70% of a
dose is recovered in the urine in 48 hours.
In
contrast, propylthiouracil is rapidly absorbed, reaching peak serum levels
after 1 hour. The bioavailability of 50–80% may be due to incomplete absorption
or a large first-pass effect in the liver. The volume of distribution
approximates total body water with accumulation in the thyroid gland. Most of
an ingested dose of propylthiouracil is excreted by the kidney as the inactive
glucuronide within 24 hours.
The short plasma
half-life of these agents (1.5 hours for propyl-thiouracil and 6 hours for
methimazole) has little influence on the duration of the antithyroid action or
the dosing interval because both agents are accumulated by the thyroid gland.
For propyl-thiouracil, giving the drug every 6–8 hours is reasonable since a
single 100 mg dose can inhibit iodine organification by 60% for 7 hours. Since
a single 30 mg dose of methimazole exerts an anti-thyroid effect for longer
than 24 hours, a single daily dose is effec-tive in the management of mild to
severe hyperthyroidism.
Both thioamides cross
the placental barrier and are concen-trated by the fetal thyroid, so that
caution must be employed when using these drugs in pregnancy. Because of the
risk of fetal hypothyroidism, both thioamides are classified as Food and Drug
Administration pregnancy category D (evidence of human fetal risk based on
adverse reaction data from investigational or market-ing experience). Of the
two, propylthiouracil is preferable during the first trimester of pregnancy
because it is more strongly protein-bound and, therefore, crosses the placenta
less readily. In addition, methimazole has been, albeit rarely, associated with
congenital malformations. Both thioamides are secreted in low concentrations in
breast milk but are considered safe for the nursing infant.
Pharmacodynamics
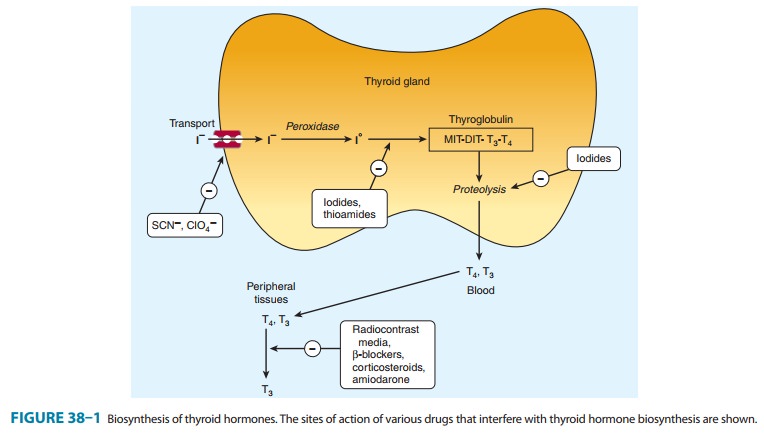
The thioamides act by
multiple mechanisms. The major action is to prevent hormone synthesis by
inhibiting the thyroid peroxi-dase-catalyzed reactions and blocking iodine
organification. In addition, they block coupling of the iodotyrosines. They do
not block uptake of iodide by the gland. Propylthiouracil and (to a much lesser
extent) methimazole inhibit the peripheral deiodina-tion of T4 and T3 (Figure 38–1). Since
the synthesis rather than the release of hormones is affected, the onset of
these agents is slow, often requiring 3–4 weeks before stores of T4 are depleted.
Toxicity
Adverse
reactions to the thioamides occur in 3–12% of treated patients. Most reactions
occur early, especially nausea and gastro-intestinal distress. An altered sense
of taste or smell may occur with methimazole. The most common adverse effect is
a maculo-papular pruritic rash (4–6%), at times accompanied by systemic signs
such as fever. Rare adverse effects include an urticarial rash,vasculitis, a
lupus-like reaction, lymphadenopathy, hypopro-thrombinemia, exfoliative
dermatitis, polyserositis, and acute arthralgia. An increased risk of severe
hepatitis, sometimes result-ing in death, has been reported with
propylthiouracil (black box warning), so it should be avoided in children and
adults unless no other options are available. Cholestatic jaundice is more
common with methimazole than propylthiouracil. Asymptomatic eleva-tions in
transaminase levels can also occur.
The
most dangerous complication is agranulocytosis (granulo-cyte count < 500
cells/mm3), an infrequent but potentially
fatal adverse reaction. It occurs in 0.1–0.5% of patients taking thioam-ides,
but the risk may be increased in older patients and in those receiving more
than 40 mg/d of methimazole. The reaction is usu-ally rapidly reversible when
the drug is discontinued, but broad-spectrum antibiotic therapy may be
necessary for complicating infections. Colony-stimulating factors (eg, G-CSF;)
may hasten recovery of the granulocytes. The cross-sensitivity between
propylthiouracil and methimazole is about 50%; there-fore, switching drugs in
patients with severe reactions is not recommended.
Related Topics