Chapter: Basic & Clinical Pharmacology : Thyroid &Antithyroid Drugs
Hyperthyroidism
HYPERTHYROIDISM
Hyperthyroidism
(thyrotoxicosis) is the clinical syndrome that results when tissues are exposed
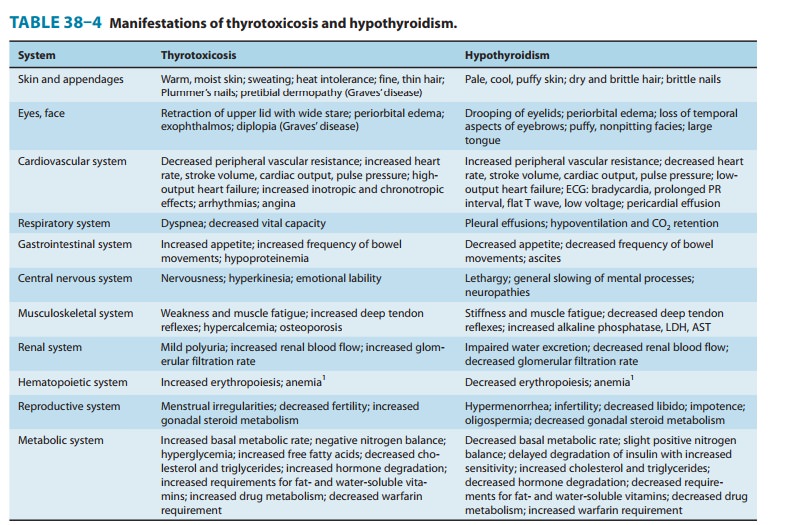
to high levels of thyroid hormone (Table 38–4).
GRAVES’ DISEASE
The most common form
of hyperthyroidism is Graves’ disease, or diffuse toxic goiter. The presenting
signs and symptoms of Graves’ disease are set forth in Table 38–4.
Pathophysiology
Graves’ disease is
considered to be an autoimmune disorder in which helper T lymphocytes stimulate
B lymphocytes to synthe-size antibodies to thyroidal antigens. The antibody
described pre-viously (TSH-R Ab [stim]) is directed against the TSH receptor
site in the thyroid cell membrane and has the capacity to stimulate growth and
biosynthetic activity of the thyroid cell. Spontaneous remission occurs but
some patients require years of antithyroid therapy.
Laboratory Diagnosis
In most patients with
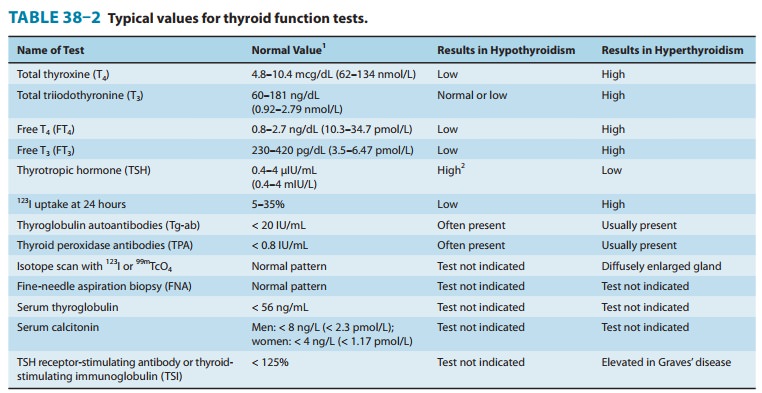
hyperthyroidism, T3, T4, FT4, and FT3 are elevated and TSH is suppressed (Table 38–2). Radioiodine
uptake is usually markedly elevated as well. Antithyroglobulin, thyroid
peroxidase, and TSH-R Ab [stim] antibodies are usually present.
Management of Graves’ Disease
The three primary methods
for controlling hyperthyroidism are antithyroid drug therapy, surgical
thyroidectomy, and destruction of the gland with radioactive iodine.
A. Antithyroid Drug Therapy
Drug therapy is most
useful in young patients with small glands and mild disease. Methimazole or
propylthiouracil is administered until the disease undergoes spontaneous
remission. This is the only therapy that leaves an intact thyroid gland, but it
does require a long period of treatment and observation (12–18 months), and
there is a 50–70% incidence of relapse.Methimazole is preferable to
propylthiouracil (except in preg-nancy and thyroid storm) because it has a
lower risk of serious liver injury and can be administered once daily, which
may enhance adherence. Antithyroid drug therapy is usually begun with divided
doses, shifting to maintenance therapy with single daily doses when the patient
becomes clinically euthyroid. However, mild to moderately severe thyrotoxicosis
can often be controlled with methimazole given in a single morning dose of
20–40 mg initially for 4–8 weeks to normalize hormone levels. Maintenance
therapy requires 5–15 mg once daily. Alternatively, therapy is started with
propylthiouracil, 100–150 mg every 6 or 8 hours until the patient is euthyroid,
followed by gradual reduc-tion of the dose to the maintenance level of 50–150
mg once daily. In addition to inhibiting iodine organification,
propylthiouracil also inhibits the conversion of T4 to T3, so it brings the level of activated thyroid
hormone down more quickly than does methi-mazole. The best clinical guide to
remission is reduction in the size of the goiter. Laboratory tests most useful
in monitoring the course of therapy are serum FT3, FT4, and TSH levels.
Reactions to
antithyroid drugs have been described above. A minor rash can often be
controlled by antihistamine therapy. Because the more severe reaction of
agranulocytosis is often her-alded by sore throat or high fever, patients
receiving antithyroid drugs must be instructed to discontinue the drug and seek
imme-diate medical attention if these symptoms develop. White cell and
differential counts and a throat culture are indicated in such cases, followed
by appropriate antibiotic therapy. Treatment should also be stopped if
significant elevations in transaminases occur.
B. Thyroidectomy
A near-total
thyroidectomy is the treatment of choice for patients with very large glands or
multinodular goiters. Patients are treated with antithyroid drugs until
euthyroid (about 6 weeks). In addition, for 10–14 days prior to surgery, they
receive saturated solution of potassium iodide, 5 drops twice daily, to
diminish vascularity of the gland and simplify surgery. About 80–90% of
patients will require thyroid supplementation following near-total
thyroidectomy.
C. Radioactive Iodine
Radioiodine therapy
utilizing 131I is the preferred
treatment for most patients over 21 years of age. In patients without heart
dis-ease, the therapeutic dose may be given immediately in a range of 80–120
μCi/g of estimated thyroid weight corrected for uptake. In patients with
underlying heart disease or severe thyrotoxicosis and in elderly patients, it
is desirable to treat with antithyroid drugs (preferably methimazole) until the
patient is euthyroid. The medication is then stopped for 5–7 days before the
appropriate dose of 131I is administered. Iodides should be avoided to ensure maximal 131I uptake. Six to 12
weeks following the administration of radioiodine, the gland will shrink in
size and the patient will usually become euthyroid or hypothyroid. A second
dose may be required in some patients. Hypothyroidism occurs in about 80% of
patients following radioiodine therapy. Serum FT4 and TSH levels should be monitored regularly.
When hypothyroidismdevelops, prompt replacement with oral levothyroxine, 50–150
mcg daily, should be instituted.
D. Adjuncts to Antithyroid Therapy
During the acute phase
of thyrotoxicosis, β-adrenoceptor–blocking
agents without intrinsic sympathomimetic activity are extremely helpful.
Propranolol, 20–40 mg orally every 6 hours, or meto-prolol, 25–50 mg orally
every 6–8 hours, will control tachycardia, hypertension, and atrial
fibrillation. Beta-adrenoceptor–blocking agents are gradually withdrawn as
serum thyroxine levels return to normal. Diltiazem, 90–120 mg three or four
times daily, can be used to control tachycardia in patients in whom β blockers are
contraindicated, eg, those with asthma. Other calcium channel blockers may not
be as effective as diltiazem. Adequate nutrition and vitamin supplements are
essential. Barbiturates accelerate T4 breakdown (by hepatic enzyme induction) and
may be helpful both as sedatives and to lower T4 levels. Bile acid sequestrants (eg,
cholestyramine) can also rapidly lower T4 levels by increasing the fecal excretion of T4.
TOXIC UNINODULAR GOITER & TOXIC MULTINODULAR GOITER
These
forms of hyperthyroidism occur often in older women with nodular goiters. FT4
is moderately elevated or occasionally nor-mal, but FT3
or T3 is strikingly elevated. Single
toxic adenomas can be managed with either surgical excision of the adenoma or
with radioiodine therapy. Toxic multinodular goiter is usually associated with
a large goiter and is best treated by preparation with methimazole (preferable)
or propylthiouracil followed by subtotal thyroidectomy.
SUBACUTE THYROIDITIS
During
the acute phase of a viral infection of the thyroid gland, there is destruction
of thyroid parenchyma with transient release of stored thyroid hormones. A
similar state may occur in patients with Hashimoto’s thyroiditis. These
episodes of transient thyro-toxicosis have been termed spontaneously resolving hyperthyroidism. Supportive therapy is
usually all that is necessary, such as β-adrenoceptor–blocking agents
without intrinsic sympathomi-metic activity (eg, propranolol) for tachycardia
and aspirin or nonsteroidal anti-inflammatory drugs to control local pain and
fever. Corticosteroids may be necessary in severe cases to control the
inflammation.
SPECIAL PROBLEMS
Thyroid Storm
Thyroid
storm, or thyrotoxic crisis, is sudden acute exacerbation of all of the
symptoms of thyrotoxicosis, presenting as a life-threatening syndrome. Vigorous
management is mandatory. Propranolol, 1–2 mg slowly intravenously or 40–80 mg
orallyevery 6 hours, is helpful to control the severe cardiovascular
manifestations. If propranolol is contraindicated by the presence of severe
heart failure or asthma, hypertension and tachycardia may be controlled with
diltiazem, 90–120 mg orally three or four times daily or 5–10 mg/h by
intravenous infusion (asthmatic patients only). Release of thyroid hormones
from the gland is retarded by the administration of saturated solution of
potassium iodide, 10 drops orally daily. Hormone synthesis is blocked by the
administration of propylthiouracil, 250 mg orally every 6 hours. If the patient
is unable to take propylthiouracil by mouth, a rectal formulation∗ can be prepared and administered
in a dosage of 400 mg every 6 hours as a retention enema. Methimazole may also
be prepared for rectal administration in a dose of 60 mg daily. Hydrocortisone,
50 mg intravenously every 6 hours, will protect the patient against shock and
will block the conversion of T4
to T3, rapidly bringing down the level
of thyroactive material in the blood.
Supportive
therapy is essential to control fever, heart failure, and any underlying
disease process that may have precipitated the acute storm. In rare situations,
where the above methods are not adequate to control the problem, plasmapheresis
or peritoneal dialysis has been used to lower the levels of circulating
thyroxine.
Ophthalmopathy
Although severe
ophthalmopathy is rare, it is difficult to treat. Management requires effective
treatment of the thyroid disease, usually by total surgical excision or 131I ablation of the
gland plus oral prednisone therapy . In addition, local therapy may be
necessary, eg, elevation of the head to diminish periorbital edema and
artificial tears to relieve corneal drying due to exophthalmos. Smoking
cessation should be advised to prevent progression of the ophthalmopathy. For
the severe, acute inflam-matory reaction, a short course of prednisone, 60–100
mg orally daily for about a week and then 60–100 mg every other day, taper-ing
the dose over a period of 6–12 weeks, may be effective. If steroid therapy
fails or is contraindicated, irradiation of the poste-rior orbit, using
well-collimated high-energy x-ray therapy, will frequently result in marked
improvement of the acute process. Threatened loss of vision is an indication
for surgical decompres-sion of the orbit. Eyelid or eye muscle surgery may be
necessary to correct residual problems after the acute process has subsided.
Dermopathy
Dermopathy or
pretibial myxedema will often respond to topical corticosteroids applied to the
involved area and covered with an occlusive dressing.
Thyrotoxicosis during Pregnancy
Ideally, women in the
childbearing period with severe disease should have definitive therapy with131I or subtotal
thyroidectomyprior to pregnancy in
order to avoid an acute exacerbation of thedisease during pregnancy or
following delivery. If thyrotoxicosis does develop during pregnancy, radioiodine
is contraindicated because it crosses the placenta and may injure the fetal
thyroid. Propylthiouracil (fewer teratogenic risks than methimazole) can be
given in the first trimester, and then methimazole can be given for the
remainder of the pregnancy in order to avoid potential liver damage. The dosage
of propylthiouracil must be kept to the minimum necessary for control of the
disease (ie, < 300 mg/d), because it may affect the function of the fetal
thyroid gland. Alternatively, a subtotal thyroidectomy can be safely performed
during the mid trimester. It is essential to give the patient a thy-roid
supplement during the balance of the pregnancy.
Neonatal Graves’ Disease
Graves’ disease may
occur in the newborn infant, either due to passage of maternal TSH-R Ab [stim]
through the placenta, stimulating the thyroid gland of the neonate, or to
genetic trans-mission of the trait to the fetus. Laboratory studies reveal an
elevated free T4, a markedly elevated
T3, and a low TSH—in
contrast to the normal infant, in whom TSH is elevated at birth. TSH-R Ab
[stim] is usually found in the serum of both the child and the mother.
If caused by maternal
TSH-R Ab [stim], the disease is usually self-limited and subsides over a period
of 4–12 weeks, coinciding with the fall in the infant’s TSH-R Ab [stim] level.
However, treatment is necessary because of the severe metabolic stress the
infant experiences. Therapy includes propylthiouracil in a dose of 5–10 mg/kg/d
in divided doses at 8-hour intervals; Lugol’s solu-tion (8 mg of iodide per
drop), 1 drop every 8 hours; and propra-nolol, 2 mg/kg/d in divided doses.
Careful supportive therapy is essential. If the infant is very ill, oral
prednisone, 2 mg/kg/d in divided doses, will help block conversion of T4 to T3. These medications
are gradually reduced as the clinical picture improves and can be discontinued
by 6–12 weeks.
SUBCLINICAL HYPERTHYROIDISM
Subclinical
hyperthyroidism is defined as a suppressed TSH level (below the normal range)
in conjunction with normal thyroid hormone levels. Cardiac toxicity (eg, atrial
fibrillation), espe-cially in older persons, is of greatest concern. The
consensus of thyroid experts concluded that hyperthyroidism treatment is
appropriate in those with TSH less than 0.1 mIU/L, while close monitoring of
the TSH level is appropriate for those with less TSH suppression.
Amiodarone-Induced Thyrotoxicosis
In addition to those
patients who develop hypothyroidism caused by amiodarone, approximately 3% of
patients receiving this drug will develop hyperthyroidism instead. Two types of
amiodarone-induced thyrotoxicosis have been reported: iodine-induced (type I),
which often occurs in persons with underlying thyroid disease (eg, multinodular
goiter); and an inflammatory thyroiditis(type II) that occurs
in patients without thyroid disease due to leak-age of thyroid hormone into the
circulation. Treatment of type I requires therapy with thioamides, while type
II responds best to glucocorticoids. Since it is not always possible to
differentiate between the two types, thioamides and glucocorticoids are often
administered together. If possible, amiodarone should be discontin-ued;
however, rapid improvement does not occur due to its long half-life.
Related Topics