Limitations | Atomic Structure | Chapter 12 | 8th Science - ThomsonŌĆÖs Atom Model | 8th Science : Chapter 12 : Atomic Structure
Chapter: 8th Science : Chapter 12 : Atomic Structure
ThomsonŌĆÖs Atom Model
ThomsonŌĆÖs Atom Model
Thomson, an English scientist,
proposed the famous atom model in the year 1904, just after the discovery of
electrons.

Thomson proposed that the shape of
an atom resembles a sphere having a radius of the order of 10-10 m.
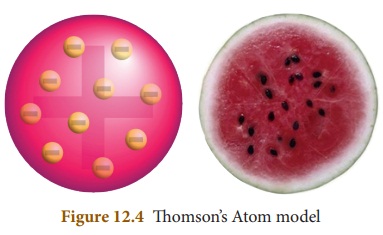
The positively charged particles are uniformly distributed with electrons
arranged in such a manner that the atom is electrically neutral. ThomsonŌĆÖs atom
model was also called as the plum pudding model or the watermelon model. The
embedded electrons resembled the seed of watermelon while the watermelonŌĆÖs red
mass represented the positive charge distribution. The plum pudding atomic
theory assumed that the mass of an atom is uniformly distributed all over the
atom.
Limitations of ThomsonŌĆÖs Atom model
ThomsonŌĆÖs atom model could
successfully explain the electrical neutrality of atom. However, it failed to
explain the following.
1. ThomsonŌĆÖs model failed to explain
how the positively charged sphere is shielded from the negatively charged
electrons without getting neutralised.
2. This theory explains only about
the protons and electrons and failed to explain the presence of neutral
particle neutron.
Related Topics