Chapter: Basic & Clinical Pharmacology : Drug Biotransformation
The Role of Biotransformation In Drug Disposition
THE ROLE OF BIOTRANSFORMATION IN
DRUG DISPOSITION
Most
metabolic biotransformations occur at some point between absorption of the drug
into the general circulation and its renal elimination. A few transformations
occur in the intestinal lumen or intestinal wall. In general, all of these
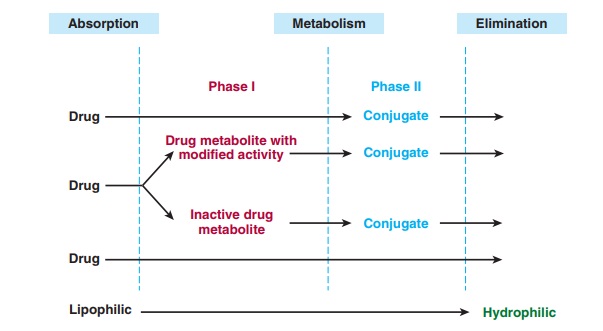
reactions can be assigned to one of two major categories called phase I and phase IIreactions (Figure 4–1).
Phase
I reactions usually convert the parent drug to a more polar metabolite by
introducing or unmasking a functional group (−OH, −NH2, −SH). Often these metabolites are inactive,
although in some instances activity is only modified or even enhanced.
If
phase I metabolites are sufficiently polar, they may be readily excreted.
However, many phase I products are not eliminated rapidly and undergo a
subsequent reaction in which an endoge-nous substrate such as glucuronic acid,
sulfuric acid, acetic acid, or an amino acid combines with the newly
incorporated functional group to form a highly polar conjugate. Such
conjugation or syn-thetic reactions are the hallmarks of phase II metabolism. A
great variety of drugs undergo these sequential biotransformation reac-tions,
although in some instances the parent drug may already possess a functional
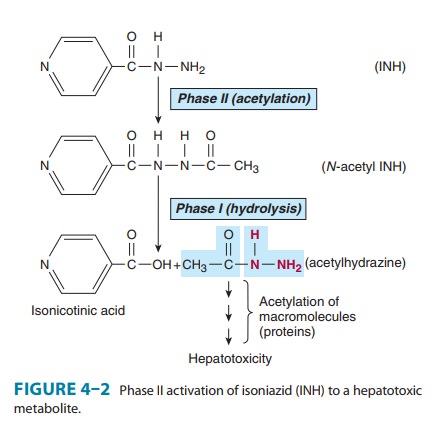
group that may form a conjugate directly. For example, the hydrazide moiety of
isoniazid is known to form an N-acetyl
conjugate in a phase II reaction. This conjugate is then asubstrate for a phase
I type reaction, namely, hydrolysis to isonico-tinic acid (Figure 4–2). Thus,
phase II reactions may actually precede phase I reactions.
Related Topics