Chapter: Basic & Clinical Pharmacology : Drug Biotransformation
Metabolism of Drugs to Toxic Products
METABOLISM OF DRUGS TO TOXIC
PRODUCTS
Metabolism
of drugs and other foreign chemicals may not always be an innocuous biochemical
event leading to detoxification and elimination of the compound. Indeed, as
previously noted, several compounds have been shown to be metabolically
transformed to reactive intermediates that are toxic to various organs. Such
toxic reactions may not be apparent at low levels of exposure to parent
compounds when alternative detoxification mechanisms are not yet overwhelmed or
compromised and when the availability of endog-enous detoxifying cosubstrates
(GSH, glucuronic acid, sulfate) is not limited. However, when these resources
are exhausted, the toxic pathway may prevail, resulting in overt organ toxicity
or carcinogenesis. The number of specific examples of such drug-induced
toxicity is expanding rapidly. An example is acetamino-phen
(paracetamol)-induced hepatotoxicity (Figure 4–5). Acetaminophen, an analgesic
antipyretic drug, is quite safe in therapeutic doses (1.2 g/d for an adult). It
normally undergoes glucuronidation and sulfation to the corresponding
conjugates, which together make up 95% of the total excreted metabolites. The alternative
P450-dependent GSH conjugation pathway
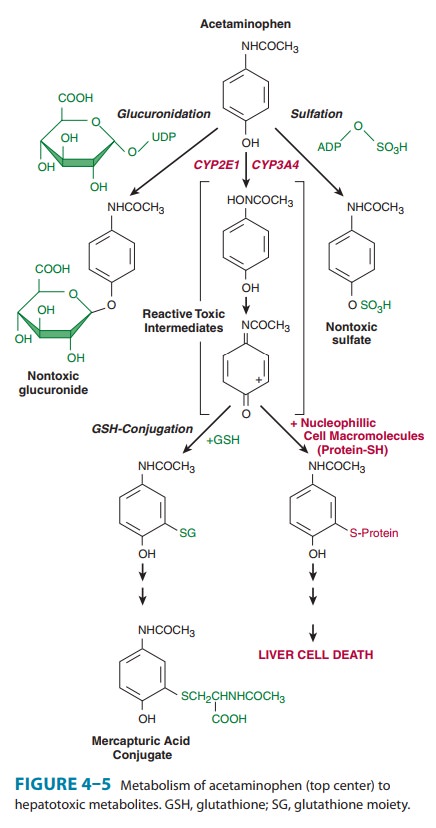
accounts
for the remaining 5%. When acetaminophen intake far exceeds therapeutic doses,
the glucuronidation and sulfation path-ways are saturated, and the P450-dependent
pathway becomes increasingly important. Little or no hepatotoxicity results as
long as hepatic GSH is available for conjugation. However, with time, hepatic
GSH is depleted faster than it can be regenerated, and a reactive, toxic
metabolite accumulates. In the absence of intracel-lular nucleophiles such as
GSH, this reactive metabolite (N-acetylbenzoiminoquinone)
reacts with nucleophilic groups of cellular proteins, resulting in
hepatotoxicity.The chemical and toxicologic characterization of the electro-philic
nature of the reactive acetaminophen metabolite has led to the development of
effective antidotes—cysteamine and N-acetylcysteine.
Administration of N-acetylcysteine
(the safer of the two) within 8–16 hours after acetaminophen overdosage has
been shown to protect victims from fulminant hepatotoxicity and death .
Administration of GSH is not effective because it does not cross cell membranes
readily.
Related Topics