Chapter: Basic & Clinical Pharmacology : Drug Biotransformation
Human Liver P450 Enzymes
HUMAN LIVER P450 ENZYMES
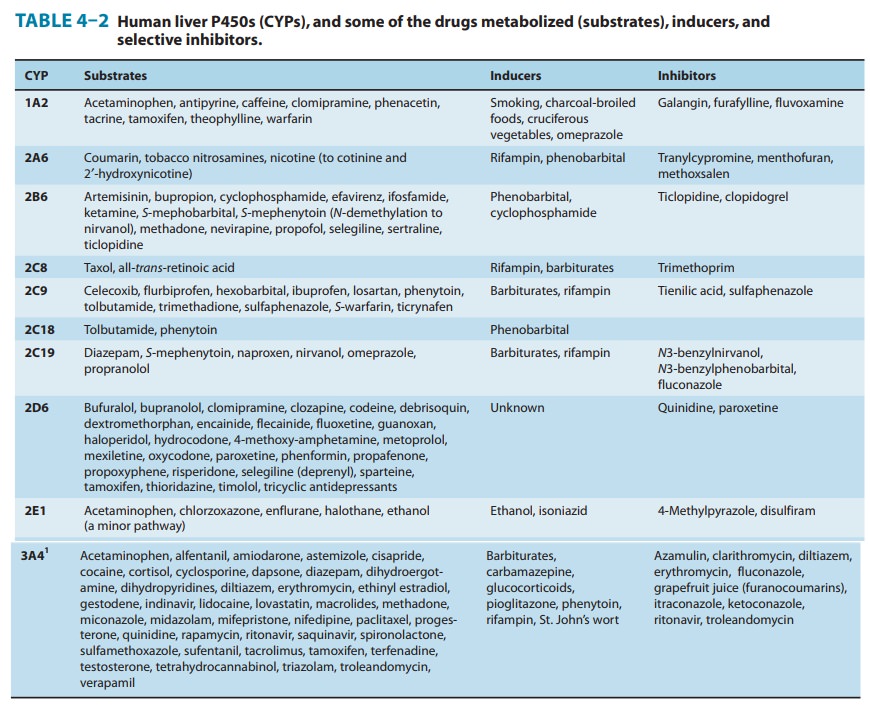
Gene arrays combined with immunoblotting analyses of microsomal preparations, as well as the use of relatively selective functional markers and selective P450 inhibitors, have identified numerous P450 isoforms (CYP: 1A2, 2A6, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5, 4A11, and 7) in the human liver. Of these, CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2D6, CYP2E1, and CYP3A4 appear to be the most important forms, accounting for approximately 15%, 4%, 1%, 20%, 5%, 10%, and 30%, respectively, of the total human liver P450 content. Together, they
are
responsible for catalyzing the bulk of the hepatic drug and xenobiotic
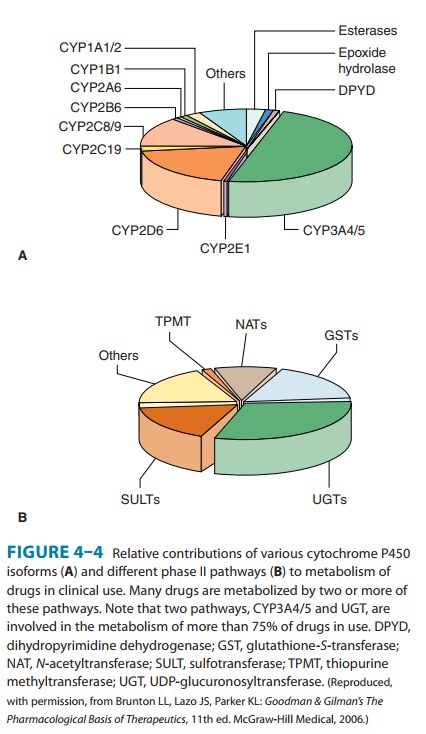
metabolism (Table 4–2, Figure 4–4).It is noteworthy that CYP3A4 alone is
responsible for the metabolism of over 50% of the prescription drugs
metabolized by the liver. The involvement of individual P450s in the metabolism
of a given drug may be screened in vitro by means of selective functional markers,
selective chemical P450 inhibitors, and P450 antibodies. In vivo, such
screening may be accomplished by meansof relatively selective noninvasive
markers, which include breath tests or urinary analyses of specific metabolites
after administra-tion of a P450-selective substrate probe.
Enzyme Induction
Some
of the chemically dissimilar P450 substrate drugs, on repeated administration, induce P450 expression by enhancing the
rate of its synthesis or reducing its rate of degradation (Table 4–2).
Induction results in accelerated substrate metabolism and usually in a decrease
in the pharmacologic action of the inducer and also of co-administered drugs.
However, in the case of drugs meta-bolically transformed to reactive
metabolites, enzyme induction may exacerbate metabolite-mediated toxicity.
Various
substrates induce P450 isoforms having different molecular masses and
exhibiting different substrate specificities and immunochemical and spectral
characteristics.Environmental chemicals and pollutants are also capable of
inducing P450 enzymes. As previously noted, exposure to benzo[a]pyrene and other polycyclic aromatic
hydrocarbons, which are present in tobacco smoke, charcoal-broiled meat, and
other organic pyrolysis products, is known to induce CYP1A enzymes and to alter
the rates of drug metabolism. Other environ-mental chemicals known to induce
specific P450s include the polychlorinated biphenyls (PCBs), which were once
used widely in industry as insulating materials and plasticizers, and
2,3,7,8-tetrachlorodibenzo-p-dioxin
(dioxin, TCDD), a trace byproduct of the chemical synthesis of the defoliant
2,4,5-T .
Increased
P450 synthesis requires enhanced transcription and translation along with
increased synthesis of heme, its prosthetic cofactor. A cytoplasmic receptor
(termed AhR) for polycyclic aro-matic hydrocarbons (eg, benzo[a]pyrene, dioxin) has been identi-fied.
The translocation of the inducer-receptor complex into the nucleus, followed by
ligand-induced dimerization with Arnt, a closely related nuclear protein, leads
to subsequent activation of regulatory elements of CYP1A genes, resulting in their induction. This is also the
mechanism of CYP1A induction by cruciferous vegetables, and the proton pump
inhibitor, omeprazole. A preg-nane X receptor (PXR), a member of the
steroid-retinoid-thyroid hormone receptor family, has recently been shown to
mediate CYP3A induction by various chemicals (dexamethasone, rifampin,
mifepristone, phenobarbital, atorvastatin, and hyperforin,
A similar receptor, the constitutive
androstane receptor (CAR), has been
identified for the relatively large and structurally diverse phenobarbital
class of inducers of CYP2B6, CYP2C9, and CYP3A4. Peroxisome proliferator
receptor α (PPAR-α) is yet another nuclear receptor highly expressed in liver
and kidneys, which uses lipid-lowering drugs (eg, fenofibrate and gemfibrozil)
as ligands. Consistent with its major role in the regulation of fatty acid
metabolism, PPAR-α mediates the induction of CYP4A enzymes, responsible for the
metabolism of fatty acids such as arachidonic acid and its physiologically
relevant derivatives. It is noteworthy that on binding of its particular ligand,
PXR, CAR, and PPAR-α each form heterodimers with another nuclear receptor,the
retinoid X-receptor (RXR). This heterodimer in turn binds to response elements
within the promoter regions of specific P450 genes to induce gene expression.
P450
enzymes may also be induced by substrate
stabilization, eg, decreased degradation, as is the case with
troleandomycin- or clotrimazole-mediated induction of CYP3A enzymes, the
ethanol-mediated induction of CYP2E1, and the isosafrole-mediated induction of
CYP1A2.
Enzyme Inhibition
Certain
drug substrates inhibit cytochrome P450 enzyme activity (Table 4–2).
Imidazole-containing drugs such as cimetidine and ketoconazole bind tightly to
the P450 heme iron and effectively reduce the metabolism of endogenous
substrates (eg, testosterone) or other co-administered drugs through
competitive inhibition. Macrolide antibiotics such as troleandomycin,
erythromycin, and erythromycin derivatives are metabolized, apparently by
CYP3A, to metabolites that complex the cytochrome P450 heme iron and render it
catalytically inactive. Another compound that acts through this mechanism is
the inhibitor proadifen (SKF-525-A, a constituent of St. John’s wort) in the
liver and intestinal mucosa. A similar receptor, the constitutive androstane
receptor (CAR), has been identified for the relatively large and structurally
diverse phenobarbital class of inducers of CYP2B6, CYP2C9, and CYP3A4.
Peroxisome proliferator receptor α (PPAR-α) is yet another nuclear receptor highly
expressed in liver and kidneys, which uses lipid-lowering drugs (eg,
fenofibrate and gemfibrozil) as ligands. Consistent with its major role in the
regulation of fatty acid metabolism, PPAR-α mediates the induction of CYP4A enzymes,
responsible for the metabolism of fatty acids such as arachidonic acid and its
physiologically relevant derivatives. It is noteworthy that on binding of its
particular ligand, PXR, CAR, and PPAR-α each form heterodimers with another nuclear
recep-tor, the retinoid X-receptor (RXR). This heterodimer in turn binds to
response elements within the promoter regions of specific P450 genes to induce gene expression.
Related Topics