Chapter: Basic & Clinical Pharmacology : Drugs Used in Asthma
Sympathomimetic Agents - Basic Pharmacology of Agents Used in the Treatment of Asthma
BASIC PHARMACOLOGY
OF AGENTS USED IN THE TREATMENT OF ASTHMA
The
drugs most used for management of asthma are adrenoceptor agonists, or
sympathomimetic agents (used as “relievers” or bron-chodilators) and inhaled
corticosteroids (used as “controllers” or anti-inflammatory agents). Their
basic pharmacology is presented elsewhere.
SYMPATHOMIMETIC AGENTS
The
adrenoceptor agonists have several pharmacologic actions that are important in
the treatment of asthma. They relax airway smooth muscle and inhibit release of
bronchoconstricting mediators from mast cells. They may also inhibit
microvascular leakage and increase mucociliary transport by increasing ciliary
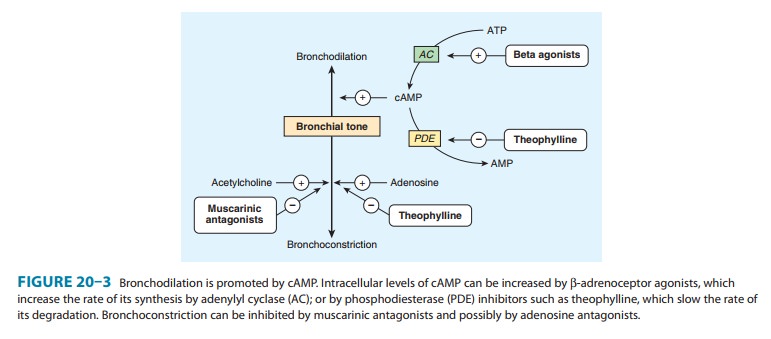
activity. As in other tissues, the β agonists stimulate adenylyl cyclase and
increase the formation of intracellular cAMP (Figure 20–3).
The
best-characterized action of the adrenoceptor agonists in the airways is
relaxation of airway smooth muscle. Although there is no evidence for direct
sympathetic innervation of human airway smooth muscle, ample evidence exists for
the presence of adreno-ceptors on airway smooth muscle. In general, stimulation
of β2
receptors
relaxes airway smooth muscle, inhibits mediator release, and causes tachycardia
and skeletal muscle tremor as side effects.The sympathomimetic agents that have
been widely used in the treatment of asthma include epinephrine, ephedrine,
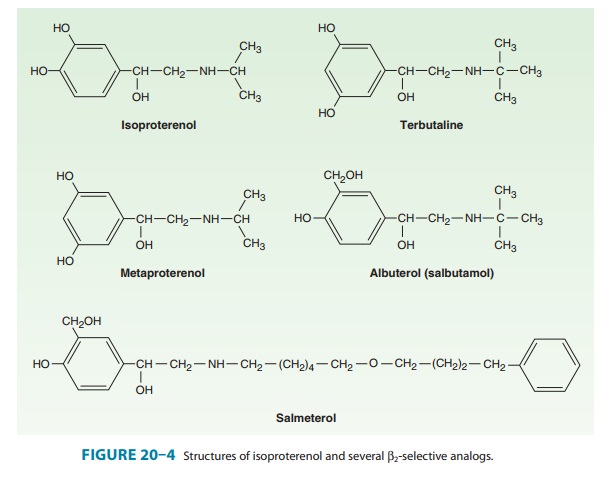
isopro-terenol, and albuterol and other β2-selective agents (Figure 20–4). Because
epinephrine and isoproterenol increase the rate and force of cardiac
contraction (mediated mainly by β1 receptors), they are reserved for special
situations .
In
general, adrenoceptor agonists are best delivered by inhala-tion because this
results in the greatest local effect on airway smooth muscle with the least
systemic toxicity. Aerosol deposi-tion depends on the particle size, the
pattern of breathing, and the geometry of the airways. Even with particles in
the optimal size range of 2–5 mm, 80–90% of the total dose of aerosol is
deposited in the mouth or pharynx. Particles under 1–2 μm remain suspended and
may be exhaled. Bronchial deposition of an aerosol is increased by slow
inhalation of a nearly full breathand by more than 5 seconds of breath-holding
at the end of inspiration.
Epinephrine is an effective, rapidly acting bronchodilatorwhen injected subcutaneously (0.4 mL of 1:1000 solution) or inhaled as a microaerosol from a pressurized canister (320 mcg per puff ). Maximal bronchodilation is achieved 15 minutes after inhalation and lasts 60–90 minutes. Because epinephrine stimu-lates α and β1 as well as β2 receptors, tachycardia, arrhythmias, and worsening of angina pectoris are troublesome adverse effects. The cardiovascular effects of epinephrine are of value for treating the acute vasodilation and shock as well as the bronchospasm of anaphylaxis, but its use in asthma has been displaced by other, more β2-selective agents. Ephedrine was used in China for more than 2000 years beforeits introduction into Western medicine in 1924. Compared with epinephrine, ephedrine has a longer duration, oral activity, more pronounced central effects, and much lower potency. Because of the development of more efficacious and β2-selective agonists, ephedrine is now used infrequently in treating asthma.
Isoproterenol is a potent bronchodilator; when inhaled as
amicroaerosol from a pressurized canister, 80–120 mcg isopro-terenol causes
maximal bronchodilation within 5 minutes. Isopro-terenol has a 60- to 90-minute
duration of action. An increase in the asthma mortality rate that occurred in
the United Kingdom in the mid-1960s was attributed to cardiac arrhythmias
resulting from the use of high doses of
inhaled isoproterenol. It is now rarely used for asthma.
Beta2-Selective Drugs
The β2-selective adrenoceptor agonist drugs, particularly albuterol, are the most widely used sympathomimetics for the treatment of the bronchoconstriction of asthma at present (Figure 20–4).
These agents
differ structurally from epinephrine in having a larger substitution on the
amino group and in the position of the hydroxyl groups on the aromatic ring.
They are effective after inhaled or oral administration and have a long
duration of action.
Albuterol, terbutaline, metaproterenol, and pirbuterol areavailable as
metered-dose inhalers. Given by inhalation, these agents cause bronchodilation
equivalent to that produced by iso-proterenol. Bronchodilation is maximal
within 15–30 minutes and persists for 3–4 hours. All can be diluted in saline
for admin-istration from a hand-held nebulizer. Because the particles
gener-ated by a nebulizer are much larger than those from a metered-dose
inhaler, much higher doses must be given (2.5–5.0 mg vs 100–400 mcg) but are no
more effective. Nebulized therapy should thus be reserved for patients unable
to coordinate inhala-tion from a metered-dose inhaler.Most preparations of β2-selective drugs are a
mixture of R and S isomers. Only the R isomer
activates theβ-agonist
receptor.Reasoning that the S isomer
may promote inflammation, a puri-fied preparation of the R isomer of albuterol has been developed (levalbuterol). Whether
this actually presents significant advan-tages in clinical use is unproven.
Albuterol
and terbutaline are also available in tablet form. One tablet two or three
times daily is the usual regimen; the principal adverse effects of skeletal
muscle tremor, nervousness, and occa-sional weakness may be reduced by starting
the patient on half-strength tablets for the first 2 weeks of therapy. This
route of administration presents no advantage over inhaled treatment and is
thus rarely prescribed.
Of
these agents, only terbutaline is available for subcutaneous injection (0.25
mg). The indications for this route are similar to those for subcutaneous
epinephrine—severe asthma requiring emergency treatment when aerosolized
therapy is not available or has been ineffective—but it should be remembered
that terbuta-line’s longer duration of action means that cumulative effects may
be seen after repeated injections.
A
new generation of long-acting β2-selective agonists includes salmeterol (a partial agonist) and formoterol (a full agonist).Both drugs
are potent selective β2 agonists that achieve their long duration of
action (12 hours or more) as a result of high lipid solubility. This permits
them to dissolve in the smooth muscle cell membrane in high concentrations or,
possibly, attach to “moor-ing” molecules in the vicinity of the adrenoceptor.
These drugs appear to interact with inhaled corticosteroids to improve asthma
control. Because they have no anti-inflammatory action, they are not
recommended as monotherapy for asthma. An ultra-long act-ing β agonist, indacaterol, is currently approved in
Europe. It needs to be taken only once a day but is used only for the
treat-ment of chronic obstructive pulmonary disease (COPD). Data on its
efficacy and safety in management of asthma are lacking.
Toxicities
The
use of sympathomimetic agents by inhalation at first raised fears about
possible cardiac arrhythmias and about hypoxemia acutely and tachyphylaxis or
tolerance when given repeatedly. It istrue that the vasodilating action of β2-agonist treatment may
increase perfusion of poorly ventilated lung units, transiently decreasing
arterial oxygen tension (PaO2). This effect is usually small,
however, and may occur with any bronchodilator drug; the significance of such
an effect depends on the initial PaO2 of the patient. Administration
of supplemental oxygen, routine in treat-ment of an acute severe attack of
asthma, eliminates any concern over this effect. The other concern, that
customary doses of β-agonist
treatment may cause lethal cardiac arrhythmias, appearsunsubstantiated. In
patients presenting for emergency treatment of severe asthma, irregularities in
cardiac rhythm improve with the
improvements in gas exchange effected by bronchodilator treat-ment and oxygen
administration.The concept that β-agonist drugs worsen clinical asthma by
inducing tachyphylaxis to their own action has not been established. Most
studies have shown only a small change in the bronchodilator response to β stimulation after
prolonged treatment with β-agonist drugs, but some studies have shown a
loss in the ability of β-agonist treatment to inhibit the response to
subsequent chal-lenge with exercise, methacholine, or antigen challenge
(referred to as a loss of bronchoprotective action).
Although
it is true that β2-adrenoceptor agonists appear to be safe and effective
bronchodilators when taken on an “as needed” basis for relief of symptoms,
there is some evidence of risk of adverse effects from chronic treatment with
long-acting β
ago-nists. These risks were suspected to be greater for individuals carrying a
genetic variant for the β receptor, specifically at the B-16 locus of
the β
receptor. Retrospective analyses of studies of regular treatment with an
inhaled β
agonist suggested that asthma control deteriorated among patients homozygous
for arginine at this locus, a genotype found in 16% of the Caucasian population
and more commonly in African Americans in the USA. It was thus tempting to
speculate that a genetic variant may underlie the report of an increase in
asthma mortality from regular use of a long-acting β agonist in studies involving very large
numbers of patients , but it should be noted that only trivial differences were
observed in multiple measures of asthma control in a study comparing patients
with the Arg/ Arg or Gly/Gly genotypes treated with salmeterol in combina-tion
with an inhaled corticosteroid. While studies so far have failed to prove that
genetic variations at this particular site in the gene for the β receptor are related
to adverse responses to pro-longed β-agonist treatment, pharmacogenetic studies of
asthma treatment will continue to be an active focus of research, as an
approach to the development of “personalized therapy” for asthma and other
diseases.
Related Topics