Heat | Chapter 4 | 8th Science - Student Activities | 8th Science : Chapter 4 : Heat
Chapter: 8th Science : Chapter 4 : Heat
Student Activities
Activity 1
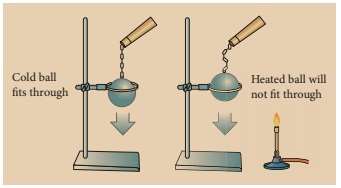
Take a metal ball and a metal ring of suitable diameter. Pass the metal ball through the ring. You can observe that the metal ball can easily go through it. Now heat the metal ball and then try to pass it through the ring. It will not pass through the ring. Keep the metal ball on the ring for some time. In few minutes, it will fall through the ring.
Activity 2
Take a cup of water and note its temperature. Heat the water for few minutes and note the temperature again.
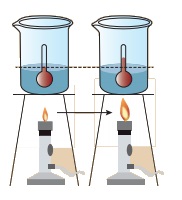
Do you find any increase in the temperature?
Answer: (i) When the water is heated, water molecules receives heat energy, increases the kinetic energy of the molecules.
What caused the temperature change?
Answer:
(i) When the molecules receive more energy, the temperature of the water increases.
(ii) Heat energy causes increase in temperature.
Activity 3
Take few ice cubes in a container and heat them for some time. What happens? The ice cubes melt and become water. Now heat the water for some time. What do you observe? The volume of water in the vessel decreases. What do you understand from this activity?
Answer:
(i) In ice cubes, the force of attraction between the water molecules is more. So they are close together.
(ii) When we heat them the force of attraction decreases and the ice cubes become water. (iii) when we heat the water, the force of attraction between the molecules decreases further.
(iv) Hence they move away from one another and become vapour.
(v) Since water vapour escape to the surrounding, water level decreases.
(vi) From this activity we understand that heat energy causes change in the state of the substances.
Activity 4
Take some hot water in a cup and put a silver spoon in it. Leave the spoon inside the water for some time. Now touch the other end of the spoon. Do you feel the heat?

Answer:
(i) Yes, we feel hot.
(ii) It is because heat in the hot water is transferred from one end to other end of the spoon.
(iii) In solid substances such as silver spoon, atoms are arranged very closely.
(iv) So heat transfer takes place from the higher temperature region to lower temperature region.
(v) This is due to conduction.
Activity 5
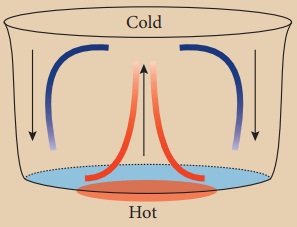
Take some water in a vessel and heat it on a stove. Touch the surface of the water. It will be cold. Touch it after some time. It will be hot now. How did the heat which was supplied at the bottom reach the top?
Answer:
(i) When water in the vessel is heated, water molecules at the bottom receive heat energy and move upward.
(ii) Then the molecules at the top comes down and get heated.
(iii) This kind of heat transfer is known as convection.
Activity 6
Take some amount of water and cooking oil in two separate vessels. Heat them till they reach a particular temperature (Caution: Heat the oil under the supervision of your teacher). Which one is heated first? Water will take more time to get heated. Why?
Answer:
(i) Heat transfer depends on the nature of the substance.
(ii) Water has high specific heat capacity than that of cooking oil.
(iii) A substance with high specific heat capacity absorbs a large quantity of heat.
(iv) Thus, it takes long time to heat up.
Electric wires used for long distance transmission of electricity will expand during day time and contract at night. That is why they will not be set very tightly. If they are set very tightly they will break when they cool at night.
Water is the only matter on the Earth that can be found naturally in all three states -Solid, Liquid and Gas.
All metals are good conductors of heat. The substances which does not conduct heat easily are called bad conductors or insulators.Wood, cork, cotton, wool, glass, rubber, etc are insulators.
Heat transfer by radiation is visible to our eyes. When a substance is heated to 500°C the radiation begins to become visible to the eye as a dull red glow, and it is sensed as warmth by the skin. Further heating rapidly increases the amount of radiation, and its perceived colour becomes orange, yellow and finally white.
The amount of energy in food items is measured by the unit kilo calorie.
1 kilo calorie = 4200 J (Approximately).
Water has higher heat capacity than most other substances. This accounts for the use of water as common coolant.100 g of water can take away more heat than 100 g of oil.
The world’s first ice-calorimeter was used in the year 1782 by Antoine Lavoisier and Pierre-Simon Laplace, to determine the heat generated by various chemical changes.
Related Topics