Heat | Chapter 4 | 8th Science - Calorimetry | 8th Science : Chapter 4 : Heat
Chapter: 8th Science : Chapter 4 : Heat
Calorimetry
Calorimetry
We studied about the effects of heat
energy. When heat energy is supplied to substances, physical changes take place
in them. Solid form of water (ice) is changed to liquid form, and liquid form
of water is changed to gaseous form. These are all the physical changes due to
heat energy. Similarly, heat energy produces chemical changes also. To know
more about the physical and chemical changes that take place in substances, we
need to measure the amount of heat involved. The technique used to measure the
amount of heat involved in a physical or a chemical process is known as
calorimetry.
1. Temperature
Temperature is a physical quantity
which expresses whether an object is hot or cold. It is measured with the help
of thermometer. There are three scales to measure the temperature. They are:
* Celcius scale
* Fahrenheit scale
* Kelvin scale
Among these three scales, Kelvin
scale is the most commonly used one. You will study about this elaborately in
Standard IX.
2. Unit of Heat
We know that heat is a form of
energy. The unit of energy in SI system is joule. So, heat is also measured in
joule. It is expressed by the symbol J. The most commonly used unit of heat is
calorie. One calorie is the amount of heat energy required to raise the temperature
of 1 gram of water through 1°C. The relation between calorie and joule is given
as, 1 calorie = 4. 186 J.
The amount of energy
in food items is measured by the unit kilo calorie.
1 kilo calorie = 4200 J (Approximately).
3. Heat capacity
Activity 6
Take some amount of
water and cooking oil in two separate vessels. Heat them till they reach a
particular temperature (Caution: Heat the oil under the supervision of your
teacher). Which one is heated first? Water will take more time to get heated. Why?
Answer:
(i) Heat transfer depends on the nature of the substance.
(ii) Water has high specific heat capacity than that of cooking
oil.
(iii) A substance with high specific heat capacity absorbs a
large quantity of heat.
(iv) Thus, it takes long time to heat up.
In general, the amount of heat
energy gained or lost by a substance is determined by three factors. They are:
* Mass of the substance
* Change in temperature of the
substance
* Nature of the material of the
substance
Different substances require
different amount of heat energy to reach a particular temperature. This nature
is known as heat capacity of a substance. Heat capacity is defined as the
amount of heat energy required by a substance to raise its temperature by 1°C
or 1 K. It is denoted by the symbol C'.
Heat capacity = Amount of heat
energy required (Q) / Raise in temperature (ΔT)
C' = Q / ΔT
The unit of heat capacity is cal / °C. In SI
system, it is measured in JK-1.
Water has higher heat
capacitythan most other substances.This accounts for the use of water as common
coolant.100 g of water can take away more heat than 100 g of oil.
Problem 1
The temperature of a
metal ball is 30° C. When an energy of 3000 J is supplied, its temperature
raises by 40° C. Calculate its heat capacity.
Solution
Heat capacity, C' = Q
/ ΔT
Here, Q = 3000 J
ΔT = 40°C - 30°C = 10°C
or 10 K
C' = 3000 / 10 = 300
JK-1
The heat capacity of
the metal ball is 300 JK-1.
Problem 2
The energy required to
raise the temperature of an iron ball by 1 K is 500 JK-1. Calculate
the amount of energy required to raise its temperature by 20 K.
Solution
Heat capacity, C' = Q
/ ΔT
Q=C'×ΔT
Here, C' = 500 JK-1
ΔT=20K
![]() Q =
500 × 20 = 10000 J.
Q =
500 × 20 = 10000 J.
The amount of heat
energy required is 10000 J.
4. Specific heat
capacity
When the heat capacity of a substance is expressed for unit mass, it is called specific heat capacity. Specific heat capacity of a substance is defined as the amount of heat energy required to raise the temperature of 1 kilogram of a substance by 1°C or 1 K. It is denoted by the symbol C.
Specific heat capacity = { Amount of heat energy required (Q) } / { Mass × Raise in temperature (ΔT) }
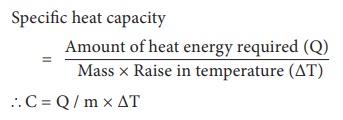
C = Q /
m × ΔT
The SI
unit of specific heat capacity is J kg-1 K -1.
Problem 3
An energy of 84000 J
is required to raise the temperature of 2 kg of water from 60° C to 70° C. Calculate
the specific heat capacity of water.
Solution
Specific heat
capacity, C = Q / m × ΔT
Here, Q = 84000 J
m = 2 kg
ΔT = 70° C – 60° C =
10° C or 10 K
C = 84000 / 2×10 = 4200 J kg-1 K-1
The Specific heat capacity of water is 4200 J kg-1 K-1.
Problem 4
The specific heat
capacity of a metal is J kg-1K-1. Calculate the amount of
heat energy required to raise the temperature of 500 gram of the metal from
125° C to 325° C.
Solution
Specific heat
capacity, C = Q / m × ΔT
Q = C × m × ΔT
Here, C = 160 J kg K-1
m = 500 g = 0.5 kg
ΔT = 325° C – 125° C =
200° C or 200 K
= 160 × 0.5 × 200 = 16000 J.
The amount of heat
energy required is 16000 J.
Related Topics