Heat | Chapter 4 | 8th Science - Questions Answers | 8th Science : Chapter 4 : Heat
Chapter: 8th Science : Chapter 4 : Heat
Questions Answers
TEXTBOOK EXERCISES
I. Choose the best answer.
1.
Heat is a form of ________.
a. electrical energy
b. gravitational energy
c. thermal energy
d. None of these
[Answer: (c) thermal energy]
2.
If you apply some heat energy to a substance, which of the following can take
place in it?
a. Expansion
b. Increase in temperature
c. Change of state
d. All the above.
[Answer: (d) All the above]
3.
Which of the following substances will absorb more heat energy?
a. Solid
b. Liquid
c. Gas
d. All the above
[Answer: (d) All the above]
4.
If you apply equal amount of heat to a solid, liquid and gas individually,
which of the following will have more expansion?
a. Solid
b. Liquid
c. Gas
d. All of them
(Answer: (c) Gas]
5.
The process of converting a liquid into a solid is called_________.
a. sublimation
b. condensation
c. freezing
d. deposition
[Answer: (c) freezing]
6.
Conduction is the way of heat transfer which takes place in a____________.
a. solid
b. liquid
c. gas
d. All of them
[Answer: (a) solid]
II. Fill in the
blanks.
1. A calorimeter is a device used to
measure the heat capacity of water.
2. Specific heat capacity is defined as the amount
of heat required to raise the temperature of 1kg of a substance by 1°C.
3. A thermostat is a device which
maintains temperature of an object constant.
4. The process of converting a
substance from gaseous state to solid state is called deposition.
5. If you apply heat energy, the temperature
of a system will increase.
6. If the temperature of a liquid in
a container is decreased, then the interatomic distance will decrease.
III. State True or False. If false, correct the statement.
1. The
applied heat energy can be realised as an increase in the average kinetic
energy of the molecules.
2. The dimensions of a substance are
increased if the temperature of the substance is decreased.
Correct statement: The dimensions of a substance are increased if the temperature of the
substance is increased.
3. The process of converting a
substance from solid state to gaseous state is called condensation.
Correct statement : The process of converting a substance from solid to gas is called sublimation.
4. Convection is the process by
which the thermal energy flows in solids.
Correct statement : Convection is the process by which the thermal energy flows in liquids and gases.
5. The amount of heat gained by a
substance is equal to the product of its mass and latent heat.
6. In a thermos flask, the silvered
walls reflect and radiate the heat outside.
Correct statement : In a thermos flask, the silvered walls reflect radiated heat back to the liquid in the bottle.
IV. Match the following.
Conduction - Liquid
Convection - Gas to liquid
Radiation - Solid to gas
Sublimation - Vaccum
Condensation - Solid
[Answer:
1 - e, 2 - a, 3 - d, 4 - c, 5 - b]
1.
Conduction (e) Solid
2.
Convection (a) Liquid
3.
Radiation (d) Gas
4.
Sublimation (c) Solid to gas
5.
Condensation (b) Gas to liquid
V. Consider the statements given below and choose the correct
option.
a. Both assertion and reason are true and reason is the correct explanation of assertion.
b. Both assertion and reason are true, but reason is not the correct explanation of assertion.
c. Assertion is true, but the reason is false.
d. Assertion is false, but the reason is true.
1. Assertion: Radiation is a form of heat transfer which takes place
only in vacuum.
Reason: The thermal energy is transferred
from one part of a substance to another part without the actual movement of the
atoms or molecules.
[Answer: (b) Both assertion and reason are true, but reason is not
the correct explanation of the assertion.]
2. Assertion: A system can be converted from one state to another state.
Reason: It takes place when the
temperature of the system is constant.
[Answer: (a) Both assertion and reason are true and the reason is
the correct explanation of the assertion.]
VI. Answer briefly.
1.
What are the applications of conduction in our daily life?
Answer:
(i) We cook food in vessels made up of metals. When the vessel
is heated, heat is transferred from the metal to the food.
(ii) When we iron dresses heat is transferred from the iron to
the cloth.
(iii) Handles of cooking utensils are made up of plastic or wood
because they are poor conductors of heat.
(iv) The temperature inside igloo (snow house) is warm because
snow is a poor conductor of heat.
2.
What are the effects of heat?
Answer:
(i) Expansion
(ii) Increase in temperature
(iii) Change in state
3.
Name three types of heat transfer.
Answer: Three types of heat transfer are :
(i) Conduction
(ii) Convection
(iii) . Radiation
4.
What is conduction?
Answer: The process of heat transfer in solids from the region of
higher temperature to the region of lower temperature without the actual
movement of atoms or molecules is called as conduction.
5.
Write a note on convection.
Answer: The form of heat transfer from places of high temperature to
places of low temperature by the actual movement of molecules is called convection. Convection takes place in
liquids and gases.
6. Define specific heat capacity.
Answer: Specific heat capacity of a substance is defined as the amount of heat
energy required to raise the temperature of 1 kilogram of a substance by 1°C or
1 K. It is denoted by the symbol C.
7.
Define one calorie.
Answer: One calorie is the amount of heat energy required to raise the
temperature of 1 gram of water through 1°C.
VII. Answer in detail.
1.
With the help of a neat diagram, explain the working of a calorimeter.
Answer:
(i) A calorimeter is a device used to measure the amount of heat
gained or lost by a substance.
(ii) It consists of a vessel made up of metals like copper or
aluminium which are good conductors of heat and electricity.
(iii) The metallic vessel is kept in an insulating jacket to
prevent heat loss to the environment.
(iv) There are two holes in it. Through one hole a thermometer
is inserted to measure the temperature of the contents.
(v) A stirrer is inserted through another hole for stirring the
content in the vessel.
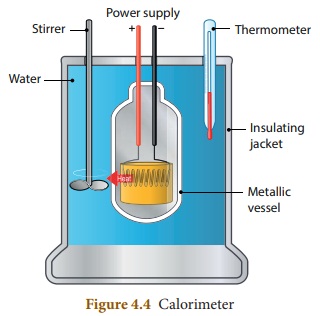
(vi) The vessel is filled with liquid which is heated by passing
current through the heating element.
(vii) Using this device we can measure the heat capacity of the liquid in the container.
2.
Write a note on thermostat.
Answer:
(i) A thermostat is a device which maintains the temperature of
a place or an object constant.
(ii) The word thermostat is derived from two Greek words,
‘thermo’ meaning heat and ‘static’ meaning staying the same.
(iii) Thermostats are used in any device or system that gets
heated or cools down - to a pre-set temperature. It turns an appliance or a
circuit on or off when a particular temperature is reached.
(iv) Devices which use thermostat include building heater,
central heater in a room, air conditioner, water heater, as well as kitchen
equipments including oven and refrigerators.
(v) Sometimes, a thermostat functions both as the sensor and the
controller of a thermal system.
3.
Explain the working of thermos flask.
Answer:
(i) A thermos flask has double walls, which are evacuated.
(ii) It is silvered on the inside.
(iii) The vacuum between the two walls prevents heat being
transferred from the inside to the outside bv conduction and convection.
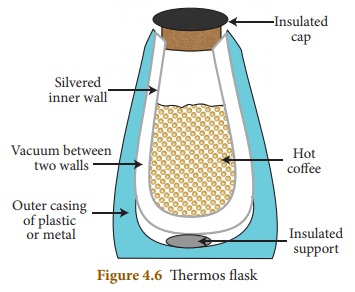
(iv) With very little air between the walls, there is almost no
transfer of heat from the inner wall to the outer wall or vice versa.
(v) Conduction can only occur at the points where the two walls
meet, at the top of the bottle and through an insulated support at the bottom.
(vi) The silvered walls reflect radiated heat back to the liquid
in the bottle.
VIII. Higher Order Thinking Questions.
1. Why
does the bottom of a lake not freeze in severe winter though the surface is all
frozen?
Answer: Lakes don’t completely freeze because the ice (and eventually
snow) on the surface acts to insulate the water below. To freeze water into
ice, a large quantity of heat is to be withdrawn. This heat cannot be supplied
all at once, so water freezes slowly and keeps the weather.
2.
Which one of the following statements about thermal conductivity is correct?
Give reason.
a) Steel > Wood > Water
b) Steel > Water > Wood
c) Water > Steel > Wood
d) Water > Wood > Steel
Answer: b) Steel > Water > Wood
Reason :
Thermal conductivity is defined as the heat flow per unit time.
Steel has a higher thermal conductivity than water and wood.
[Thermal conductivity of steel = 50.2 w/mk
Thermal conductivity of water = 0.6 w/mk
Thermal conductivity of wood = 0.12 w/mk]
IX. Numerical Problems.
1. An
iron ball requires 1000 J of heat to raise its temperature by 20°C. Calculate
the heat capacity of the ball.
Solution
:
Heat capacity C’ = Q/∆T
Here, A = 1000 J
T = 20°C − 0°C = 20°C = 20K
C = 1000/20 = 50 JK−1
The heat capacity of the ball = 50 JK−1
2.
The heat capacity of the vessel of mass 100 kg is 8000 J/°K. Find its specific
heat capacity.
Solution
:
Specific heat capacity, C = Q
/ [ m × ∆T]
Here, m = 100 kg
Heat capacity = Q / ∆T = 8000 J/°C = 8000 J/K
C = Q / [ m × ∆T] = 100 × 8000 J = 8,00,000 JKg-1K-1
Related Topics