Heat | Chapter 4 | 8th Science - Points to Remember, Glossary, Concept Map | 8th Science : Chapter 4 : Heat
Chapter: 8th Science : Chapter 4 : Heat
Points to Remember, Glossary, Concept Map
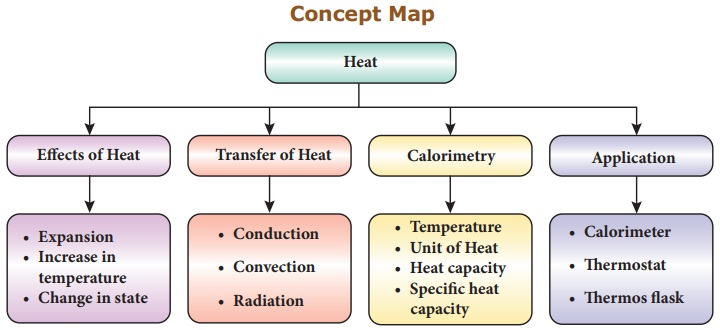
Points to Remember
• Heat is a form of energy which is
transferred from one part to another part of a substance.
• Heat transfer causes expansion,
increases temperature and changes the state of the substance.
• When thermal energy is supplied to
a solid, the atoms or molecules present in it gain energy and vibrate more
vigorously about their fixed positions, forcing each other further apart.
• Melting, vapourisation,
sublimation, condensation, freezing and deposition are the change of states
that take place due to heat energy.
• Heat transfer takes place in three
ways: conduction, convection and radiation.
• Conduction occurs in solids,
convection in liquids and gases, and radiation takes place in vaccum.
• Capacity of substances to gain or
loose heat energy is determined by three factors: mass of the substance, change
in temperature and nature of the substance.
• There are three scales to measure
temperature: Celcius scale, Fahrenheit scale and Kelvin scale.
• Calorimeter measures the heat
capacity of water.
GLOSSARY
1. Calorimeter A device which measures the heat
capacity of liquids.
2.
Calorimetry The
technique used to measure the amount of heat involved in a physical or a
chemical process.
3.
Conduction The process of heat
transfer in solids from a region of higher temperature to a region of lower temperature
without the actual movement of molecules.
4.
Convection The form of heat
transfer from places of high temperature to places of low temperature by the actual
movement of liquid or gas molecules.
5.
Heat capacity Amount
of heat energy required to raise the temperature of a substance by 1° C or 1
K.
6.
Radiation The form of heat
transfer from one place to another place in the form of electromagnetic waves.
7.
Specific heat capacity Amount
of heat energy required to raise the temperature of 1 kilogram of a substance
by 1° C or 1 K.
8.
Temperature Physical
quantity which expresses whether an object is hot or cold.
9.
Thermos flask An
insulating storage vessel that keeps its content hotter or cooler than the
surroundings for a longer time.
10.
Thermostat A
temperature sensing device that turns an appliance or circuit on or off when a
particular temperature is reached in it.
REFERENCE BOOKS
1. Fundamentals of Statistical and
Thermal Physics - F.Reif
2. Statistical Thermodynamics and
Microscale Thermo -physics - Carey
3. Heat, Thermodynamics and Statistical
Physics - BrijLal and Dr. N. Subramaniyam
4.Thermodynamics and an Introduction
to Thermos-statistics byHerbert Hallen
5.Fundamentals of Engineering Thermo
dynamics by Michael Moran
INTERNET RESOURCES
https://w w w. explainthatstuf f .
com/ thermostats.html
https://youtu.be/8-nLHWpgDsM
https://youtu.be/rYwgsF_haAg
Concept Map
ICT CORNER
Heat
Through
this activity you will learn about heat energy through Interactive games.

Step
1 Open the Browser and type the URL
given below
Step
2 You can see lot of games about heat
energy.
Step
3 For example, click “Heat Energy
match it” game. You will see the match words in the screen. Play and learn
about heat energy.
Step
4 Likewise you can explore all the
games.
Browse in the link:
https://www.learninggamesforkids.com/heat-energy-games.html
Related Topics