Heat | Chapter 4 | 8th Science - Effects of Heat | 8th Science : Chapter 4 : Heat
Chapter: 8th Science : Chapter 4 : Heat
Effects of Heat
Effects of Heat
When heat energy is supplied to any
substance, it brings about many changes. There are three important changes that
we can see in our daily life. They are:
* Expansion
* Increase in temperature
* Change in state
1. Expansion
Activity 1
Take a metal ball and
a metal ring of suitable diameter. Pass the metal ball through the ring. You
can observe that the metal ball can easily go through it. Now heat the metal
ball and then try to pass it through the ring. It will not pass through the
ring. Keep the metal ball on the ring for some time. In few minutes, it will
fall through the ring.
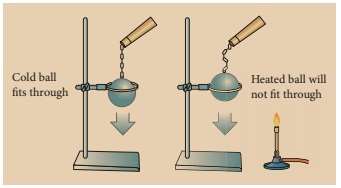
Why didn’t the ball go through the
ring initially but went through it after some time? When the ball is heated the
atoms in the ball gain heat energy. They start vibrating and force each other
apart. As a result an expansion takes place. That’s why the ball did not go
through the ring. After some time, as the ball lost the heat energy to the
surrounding it came back to its original size and it went through the ring.
This shows that heat energy causes expansion in solids. This expansion takes
place in liquids and gases also. It is maximum in gases.
Electric wires used for
long distance transmission of electricity will expand during day time and
contract at night. That is why they will not be set very tightly. If they are
set very tightly they will break when they cool at night.
2. Rise in Temperature
Activity 2
Take a cup of water and note its temperature. Heat the water for few minutes and note the temperature again.
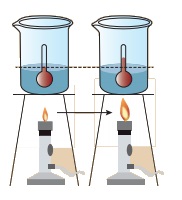
Do you find any increase in the temperature?
Answer: (i) When the water is heated, water molecules receives heat
energy, increases the kinetic energy of the molecules.
What caused the
temperature change?
Answer:
(i) When the molecules receive more energy, the temperature of the
water increases.
(ii) Heat energy causes increase in temperature.
When the water is heated, water
molecules receive heat energy. This heat energy increases the kinetic energy of
the molecules.When the molecules receive more energy, the temperature of the
water increases. This shows that heat energy causes increase in temperature.
3. Change of State
Activity 3
Take few ice cubes in
a container and heat them for some time. What happens? The ice cubes melt and
become water. Now heat the water for some time. What do you observe? The volume
of water in the vessel decreases. What do you understand from this activity?
Answer:
(i) In ice cubes, the force of attraction between the water
molecules is more. So they are close together.
(ii) When we heat them the force of attraction decreases and the
ice cubes become water. (iii) when we heat the water, the force of attraction
between the molecules decreases further.
(iv) Hence they move away from one another and become vapour.
(v) Since water vapour escape to the surrounding, water level
decreases.
(vi) From this activity we understand that heat energy causes
change in the state of the substances.
In ice cubes the force of attraction
between the water molecules is more. So they are close together. When we heat
them the force of attraction between the molecules decreases and the ice cubes
become water. When we heat the water, the force of attraction decreases
further. Hence they move away from one another and become vapour. Since water
vapour escape to the surrounding, water level decreases further. From this we
understand that heat energy causes change in the state of the substances. When
heat energy is removed, changes take place in reverse direction.
If heat energy is supplied to or
taken out from a substance, it will undergo a change from one state of matter
to another.
One of the following transformations
may take place due to heat energy.
* Solid to Liquid (Melting)
* Liquid to Gas (Vapourisation)
* Solid to Gas (Sublimation)
* Gas to Liquid (Condensation)
* Liquid to Solid (Freezing)
* Gas to Solid (Deposition)
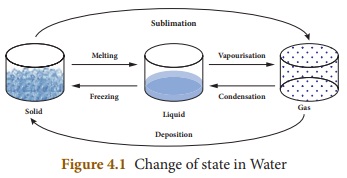
Water is the only
matter on the Earth that can be found naturally in all three states -Solid,
Liquid and Gas.
Related Topics