Chapter: Organic Chemistry: Organic spectroscopy and analysis
Spectroscopy
SPECTROSCOPY
Key Notes
Introduction
The
absorption or emission of energy from electromagnetic radiation is involved in
IR, vis/uv and nmr spectroscopy. Mass spectroscopy involves measuring the
deflection of ions in a magnetic field.
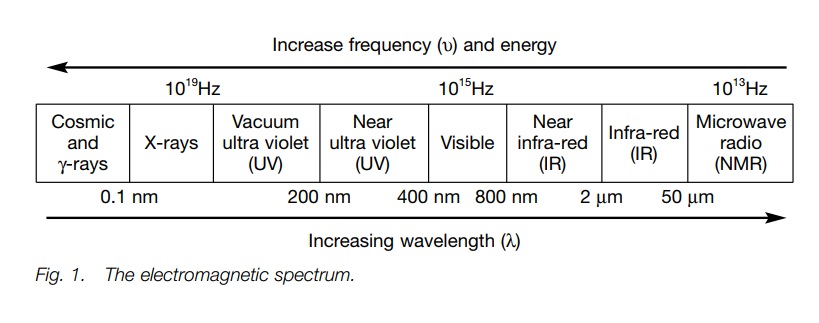
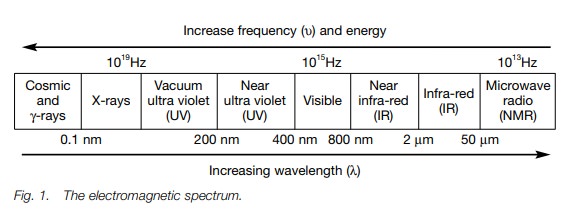
Electromagnetic radiation
Electromagnetic
radiation has the properties of both a wave and a particle. The former can be
defined by wavelength and frequency, which in turn define the energy. Energy is
proportional to frequency and inversely pro-portional to wavelength.
Introduction
There are three important spectroscopic methods
used in the analysis of organic compounds which involve the use of
electromagnetic radiation. These are visible/ultra violet (vis/uv), infra-red
(IR) and nuclear magnetic resonance (nmr) spectroscopy. These methods measure
the absorption or emission of energy from electromagnetic radiation arising
from different regions of the electromagnetic spectrum (Fig. 1). In contrast, mass spectroscopy measures the deflection of
ions in a magnetic field.
Electromagnetic radiation
Electromagnetic radiation has the properties of
both a wave and a particle. The latter can be described in terms of quanta or
photons. The former can be described by wavelength
(λ) – the distance between the crests of different waves, and frequency (υ) – the number of waves that pass a given point each
second.Frequency is measured in hertz (Hz), which is the same as cycles per
second. The energy of electromagnetic radiation is related to frequency and
wavelength by the following equation:
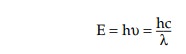
where h = Planck’s constant (6.63 × 10−34 J s−1), and c is the velocity of light (2.99792458 × 108 m s−1).
Therefore, the higher the frequency of
radiation, the higher the energy.Con-versely, the higher the wavelength, the
lower the energy. Thus in the visible spec-trum, violet light (λ = 400 nm) has a higher energy than red light (λ = 750 nm).
Related Topics