Chapter: Organic Chemistry: Organic spectroscopy and analysis
Proton nuclear magnetic resonance spectroscopy
PROTON NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
Key Notes
Secondary magnetic fields
The
presence of secondary magnetic fields in a molecule means that non-equivalent
protons experience slightly different magnetic fields when the external
magnetic field is applied. This means that different protons require different
energies for resonance and give different signals in a spectrum.
Secondary magnetic fields due to electrons
Electrons
are charged spinning bodies, which set up a secondary magnetic field that
opposes the applied field and shields the proton. The greater the electron
density around a proton, the greater the shielding and the less energy is
required for resonance.
The nmr spectrum
The
protons which give signals at the right hand side of an nmr spectrum are more
shielded than those at the left-hand side and require less energy to resonate.
The scale used in nmr is known as the chemical shift which is measured in
parts per million relative to the signal for a reference compound called
tetramethylsilane.
Inductive effects on chemical shift
The
position of a signal in the nmr spectrum is affected by the inductive effects
of various groups. Electron donating groups increase the electron density round
a neighboring proton and lower the chemical shift. Electron withdrawing groups
have the opposite effect.
Diamagnetic circulation
If an
unsaturated group is present in a molecule, it is possible to get secondary
magnetic fields due to diamagnetic circulation. This is a result of the
external magnetic field causing the π electrons to circulate around the axis of the
magnetic field. The effect is large for aromatic rings since six π electrons are involved, and
smaller for groups such as alkenes and ketones. For most unsaturated systems,
the secondary magnetic field enhances the applied magnetic field and increases
chemical shift. The protons of aldehy-des and carboxylic acids experience
secondary magnetic fields due to dia-magnetic circulation as well as electron
withdrawing inductive effects, resulting in very large chemical shifts.
Integration
Integration
measures the relative intensity of each signal in the nmr spectrum and is
proportional to the number of protons responsible for that signal.
Secondary magnetic fields
At this stage, we can see how a proton is
detected by nmr spectroscopy, but if that was
all there was
to it we
would only see
one signal for
every proton in a
molecule. This would tell us nothing about the structure apart from the fact
that protons are present.
Fortunately, not all
protons require the
same energy for resonance. This
is because there
are secondary magnetic
fields within the molecule,
which influence the
magnetic field experienced
by each proton. Secondary magnetic fields are
produced by the electrons in the molecule and are much smaller in magnitude
than the applied magnetic field – in the order of 0–10 parts per million (ppm).
However, they are sufficiently large enough to result in different signals for
different protons. This means that there should be one signalfor every
different (or non-equivalent) proton in the structure. Therefore, it is useful
to identify the number of non-equivalent protons in a molecule in order to
identify the number of signals that should be present in the spectrum. Note
that the protons in a methyl group are equivalent and do not give separate
signals because they are in identical molecular environments. This is also true
for the protons in a CH2 group. (However, there are two situations
where the two protons on a CH2
group become non equivalent, i.e. when they are constrained within a
ring system and
when they are
next to an
asymmetric center.) The
size and direction of secondary
magnetic fields depends on electron density, diamagnetic circulation and spin-spin
coupling all of which are discussed below.
Secondary magnetic fields due to electrons
Fig. 3a shows an isolated proton spinning and precessing in an applied
magnetic field. So far we have only considered the nucleus of the hydrogen
atom, but we know that electrons must be present. Let us consider the effect of
one electron orbiting the nucleus (Fig. 4).
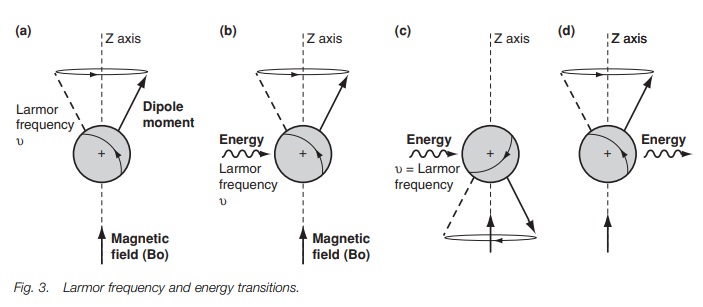
Since the electron is a spinning, charged body,
it sets up a secondary magnetic Vfield of its own (Be, Fig. 4). The secondary
magnetic field (Be) opposes the exter- nal magnetic field (Bo). Thus the
nucleus is shielded from the external magnetic field. This means the actual
magnetic field experienced by the nucleus is reduced (Bo–Be). Since the nucleus
experiences a reduced magnetic field, the precessional or Larmor frequency is
reduced. This in turn means that less energy is required to make that nucleus
resonate and give a signal. The greater the electron density round a proton,
the greater the shielding and so the position of a signal in an nmrspectrum can
be an indication of electron density in different parts of a molecule.
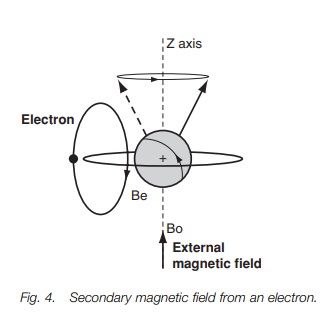
The nmr spectrum
The signals at the right hand side of an nmr
spectrum (Fig. 5) are due to protons
having a low precessional frequency while the signals at the left-hand side are
dueto protons having a high precessional frequency. Low precessional frequency
isassociated with high electron density
(shielding) while high
precessional frequency is associated
with low electron
density (deshielding). The
energyrequired for resonance increases from right to left.
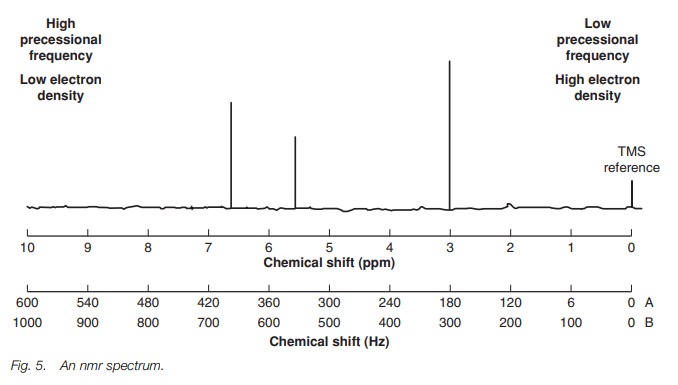
A scale is needed in order to quantify the
position of signals in an nmr spec-trum. The scale used in nmr does not have
absolute values, but is relative to the signal of a reference compound called
tetramethylsilane (TMS) (Fig. 6). The
methyl protons of TMS are equivalent and give one signal that is defined as the
zero point on the scale. The scale is known as the chemical shift and is measured
in parts per million (ppm). What does this mean and why do we not use an
absolute scale, which uses frequency or energy units?
Let us look at what happens if we measure the
chemical shift in frequency units of hertz. The scale shown in Fig. 5A is for a 60 MHz nmr
spectrometer. The 60 MHz refers to the frequency of the energy required to
cause resonance. Thus, the signal at 3 ppm has a Larmor frequency which is 180
Hz faster than the signal due to TMS (180 Hz is 3 ppm of 60 MHz).
However, we could also measure this spectrum
using a more powerful nmr spectrometer which results in the protons having
precessional frequencies in the
In this situation, the signal at 3 ppm is due to a proton
that is precessing 300 Hz faster than the protons for TMS. If we measure the
chem-ical shift of this peak in ppm, this would still be 3 ppm, since 300 Hz is
3 ppm of 100 MHz. However, if we used a scale measured in Hertz, we would have
to define the power of the nmr spectrometer used. With chemical shift measured
in parts per million, the chemical shift will be the same no matter which
instrument is used.
Inductive effects on chemicalshift
TMS is called an internal reference since it is
dissolved in the deuterated solvent used to dissolve the sample. There is a
good reason why TMS is used as an internal reference. Silicon has a tendency to
‘repel’ the electrons in the silicon-carbon bonds such that they are pushed
towards the methyl groups (Fig. 6) –
an inductive effect. This means that
the protons in these methyl groups experience ahigh electron density that
shields the nuclei and results in a low chemical shift, lower in fact than the
vast majority of nmr signals observed in organic molecules.
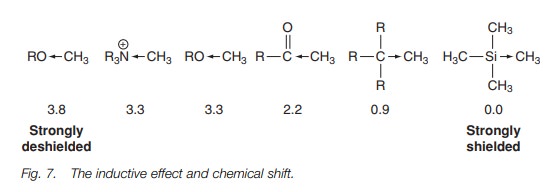
Inductive effects have an important influence
on chemical shift. We can see this with a series of methyl groups (Fig. 7). Going from right to left we
have TMS which sets the scale at 0 ppm. Next we have the methyl group of a saturated
hydrocarbon. The alkyl groups of a hydrocarbon also ‘push electrons’ away from
them and hence increase electron density round a neighboring methyl group.
However this inductive effect is not as powerful as the one caused by a silicon
atom and so the methyl signals in hydrocarbons usually occur about 0.9 ppm.
In the next case we have a methyl group next to a ketone group. A ketone group has an electron withdrawing effect, which reduces the electron density around the neighboring methyl group (deshielding), and so the chemical shift is higher at about 2.2 ppm. The electron withdrawing effect of an oxygen atom or a positively charged nitrogen is greater still and so a methyl ether group has a signal at about 3.3 ppm, similar to the methyl signal of a quaternary ammonium salt.
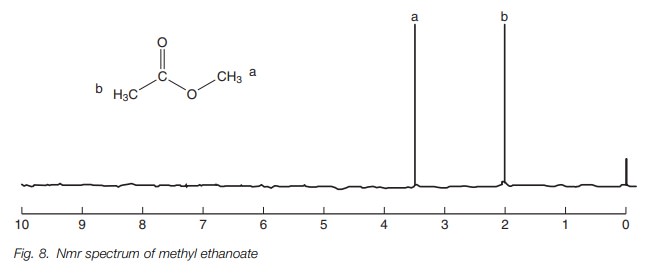
Since the position of a signal in the nmr
spectrum is related to inductive effects, it is possible to use this knowledge
to assign different signals in an nmr spectrum. For example, the nmr spectrum
for methyl ethanoate contains two signals at 2.0 and 3.5 ppm (Fig. 8). The signal at 3.5 ppm can be
assigned to CH3a since it is directly attached to oxygen.
The oxygen has a stronger electron withdrawing effect than the carbonyl group.
NMR tables are also available which show the typical chemical shifts for
particular groups.
Diamagnetic circulation
A special type of secondary magnetic effect is
called diamagnetic circulation. This occurs in functional groups which contain π bonds. As an example, we shall consider the aromatic ring (Fig. 9).
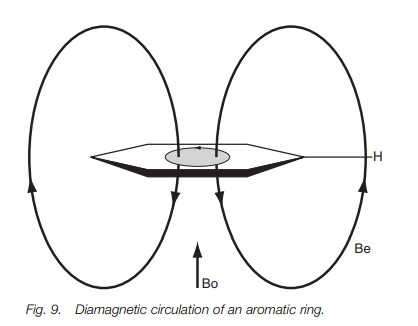
The applied external magnetic field (Bo) used
in nmr has an interesting effect on the π-electrons of the aromatic ring, causing them to circulate round
the ring in a process known as diamagnetic
circulation. This movement in turn sets up a sec-ondary magnetic field (Be)
represented by the force lines shown in Fig.
9. The direction of these lines opposes the applied magnetic field at the
center of the aro-matic ring, but enhances it at the edges where the aromatic
protons are situated. This means that the field observed by the aromatic
protons is increased (Bo+Be) causing their precessional frequency to increase.
This means that a greater energy is required for resonance, resulting in a
higher chemical shift
The effect of diamagnetic circulation on
aromatic protons is greater than inductive effects and this can be seen in the
nmr spectrum of benzyl methyl ether (Fig.
10).
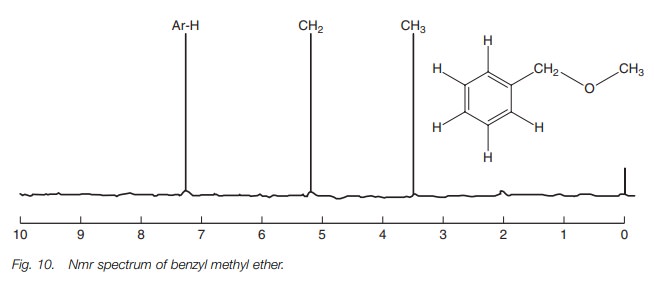
The methyl group at 3.6 ppm has a relatively
high chemical shift due to the inductive effect of oxygen. The methylene group
at 5.2 ppm has an even higher chemical shift since it is next to oxygen and the
aromatic ring, both of which are electron withdrawing groups. However, the
aromatic protons have the highest chemical shift at 7.3 ppm since they
experience the secondary magnetic field set up by diamagnetic circulation.
Diamagnetic circulation is also possible for
other unsaturated systems such as alkenes. However, the diamagnetic circulation
for an alkene is much smaller since only two π
electrons are circulating within a double bond, and so the effect is
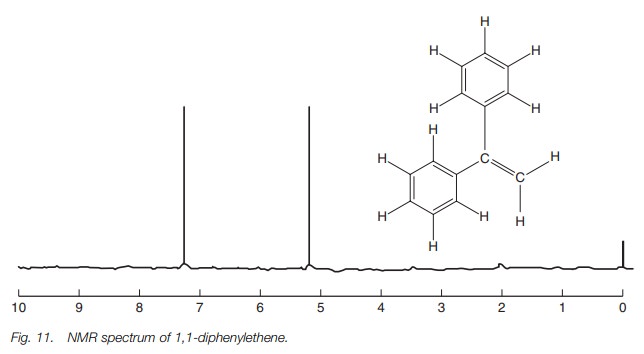
smaller. This can be seen in the nmr spectrum of 1,1-diphenylethene where the
alkene protons have a smaller chemical shift at 5.2 ppm compared to the
aromatic protons at 7.3 ppm (Fig. 11).
An aldehyde proton also experiences a secondary
magnetic field due to dia- magnetic circulation, but in addition there is an
inductive effect resulting from the electron withdrawing effect of the carbonyl
group. Thus, an aldehyde proton experiences two deshielding effects, which
means that it has a higher chemical shift than even an aromatic ring.
Typically, the signal appears about 9.6 ppm.
The combined influences of diamagnetic
circulation and inductive effects also result in high chemical shifts for the
OH of a carboxylic acid where the signal can have a chemical shift larger than
10 ppm.
For most unsaturated systems, diamagnetic circulation sets up a secondary field which enhances the applied magnetic field. The exception is alkynes where the secondary field opposes the applied field and causes shielding.
Integration
An nmr spectrum contains another piece of
useful information which is called integration.
Integration measures the intensity of each signal and is proportionalto the
number of protons responsible for that signal. Thus, a signal due to a methyl
group will be three times more intense than one due to a methine (CH) group.
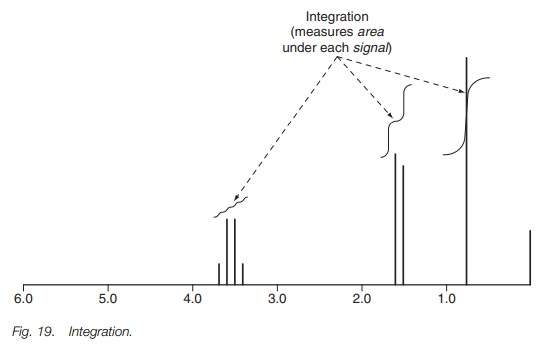
The integration signal on the nmr spectrum is
the sloping line above the signals (Fig.
19). There is no absolute scale to this line, but the relative heights of
the inte-gration over each signal are proportional to the number of protons
responsible for each signal. Note that it is the height increase over the whole signal that should be measured
(i.e. you measure the height increase over all the peaks in the coupling
pattern).
Another thing to watch out for is the
possibility of OH or NH2 groups being present in a spectrum. These
will also be integrated, but can be distinguished from CH, CH2 and
CH3 groups since the protons in the former disappear from the
spectrum if the sample is shaken with D2O.
Related Topics