Chapter: Aquaculture Engineering : Aeration and Oxygenation
Sources of oxygen
Sources of oxygen
Oxygen gas may be delivered from oxygen producers, either as compressed gas in bottles or as liquid oxygen, which the farms transforms to gas under controlled conditions. This oxygen is produced in special factories that produce only oxygen. Whether gas or liquid, commercially pro-duced oxygen is characterized by high purity (>99%) and the presence of a small amount of other gases.
The other alternative is on-site production of oxygen, which means that the oxygen is produced at the farm with air as the source.
Oxygen gas
Oxygen gas may be delivered as compressed gas in bottles. In Norway the bottles are blue and can be delivered as single bottles or in batteries, normally of 12 (Fig. 8.11). The usual pressure inside an oxygen gas bottle is as high as 200 bar; the bottle contains 50 l of compressed gas at this pressure and this gives 10 000 l gas at normal atmospheric pres-sure. On the top of the bottle there is a pressure reduction valve. A manometer, also on the top of the bottle shows the internal pressure; this also

indicates the amount of gas remaining in the bottle, because the pressure inside the bottle will decrease as oxygen is released. The bottles are normally rented from the oxygen producers, one reason being that the producer will ensure the bottles are properly maintained. The price of oxygen gas depends on the distance from the oxygen produc-tion site amongst other things. The pressurized bottles represent a real danger, for instance in con-nection with a fire. It is therefore advisable not to have too many bottles on the farm, and if possibleto place them outside; legal restrictions may limit the number of gas bottles that can be stored inside buildings.
Liquid oxygen
Liquid oxygen (LOX) is produced in special facto-ries and transported to the farms by truck or boat in special tanks. On arrival, the liquid oxygen is transferred to the farm’s specially designed tank for storage (Fig. 8.12). The usual tank size for storingliquid oxygen is between 2 and 50 m3. The oxygen is stored as liquid in the tank with a temperature below the boiling point and of a pressure of between 15 and 25 bar.

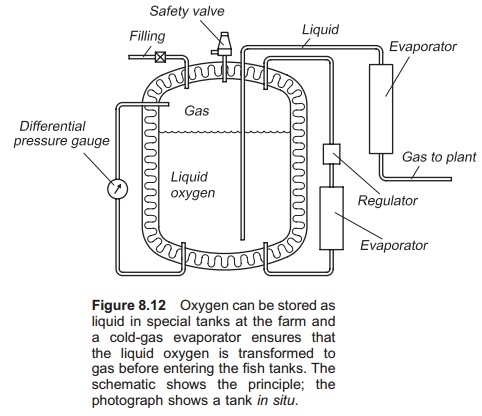
The pressure in the liquid oxygen tank will stay constant. An internal evaporator system, normally placed under the liquid tank, ensures this. Liquid oxygen is taken from the tank, goes through the evaporator where the liquid is transformed to gas, and back again as gas into the top of the tank. The expansion when oxygen goes from liquid to gas is utilized in this process and maintains the pressure in the tank. The tank is fitted with a safety valve to prevent it exploding, because even though the tank is constructed with two shells with space between them it is impossible to achieve 100% effective isolation of the tank. Therefore some liquid will always evaporate to gas inside the tank. With the large expansion in volume when changing phase from liquid to gas, the tank will easily blow if nothing is done, which is where the safety valve comes into play. If the daily use of oxygen from the tank is too low, oxygen gas will pass through the safety valve and into the atmosphere. Normally 0.1–0.6% of the tank volume must be used daily, depending on tank size, to avoid loss of oxygen through the safety valve to the atmosphere. This also shows the importance of choosing a tank of the correct size.
When the farm uses oxygen from the tank, it is drained as liquid from a pipe near the bottom of the tank. Because of the pressure in the tank, the liquid oxygen will be pressed out through the outlet pipe and into the evaporator when the outlet valve is opened. In the evaporator liquid oxygen changes phase to gas. The gas is transported from the evap-orator through a pipe and into the farm’s oxygen injection system.
The evaporator is known as a cold gas evap-orator and must be designed according to the farm’s oxygen consumption. It functions as a heat exchanger transferring heat from the air into the oxygen liquid (air–liquid exchange). It is important to have a large surface area to get a large contact area between the air and the oxygen. Usually at least two evaporators are installed, one runs while the other is switched off. The evaporator takes energy from the air and after a while is totally covered with ice; deicing is therefore necessary and is the reason for at least two evaporators being required.
The liquid oxygen tank should be placed outside on a concrete base and be fenced in. In addition, there should be a safety zone around the tank. Oxygen tanks are rented from the oxygen suppliers for safety reasons. With online measurement of the remaining oxygen content in the tank by the sup-plier, it is also possible for the supplier to automatically replenish the oxygen before the tank is empty. It is then not necessary for the fish farmer to keep control of this.
On-site oxygen production
The alternative to buying oxygen from a producer is to have on-site production facilities (Fig. 8.13). Air contains 78% nitrogen, 21% oxygen, 0.9% argon and 0.1% of other gases by volume (Table 8.1). In a specially designed adsorption unit it is possible to remove the nitrogen and some of the other gases from the air, leaving mainly oxygen. The absorption unit is fed by compressed air from a compressor; different names are used (oxygen gen-erator or xorbox) according to the producer. Put simply, it is a unit that filters air and lets only oxygen pass. The process is known as pressure swing adsorption (PSA).
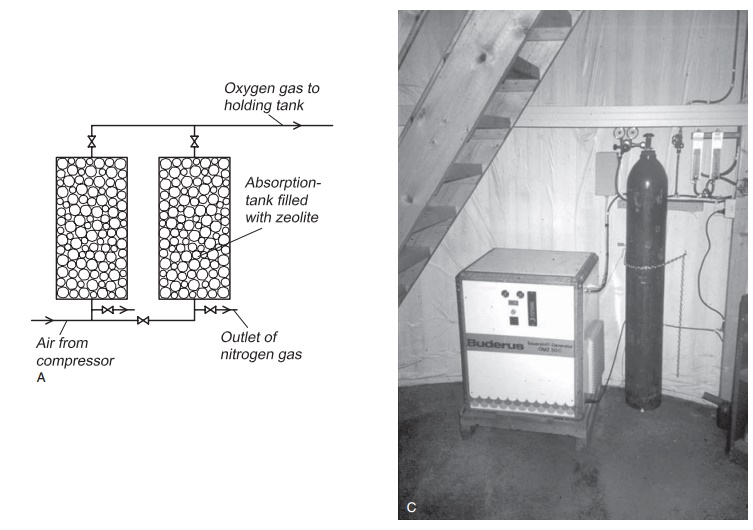

The compressed air passes into an absorption unit that is normally filled with clinoptilolite, a natural zeolite which is a clay mineral. To describe the construction and function in detail is beyond the scope of this book but a brief and simplified description is as follows. The crystals in the filter material form a latticework where the nitrogen molecules are trapped. Oxygen molecules, which are large, do not fit into the latticework and pass through; almost pure oxygen is produced. Provided that the filter medium is not contaminated with oil or water from the compressor, it is quite durable. An inlet air filter must be used and cleaned at regular intervals. A normal generator used for aquaculture can reach a purity of 95% oxygen, the rest being argon (4%) and nitrogen (1%). Special units may achieve 99% oxygen purity, but they are very expensive and not viable for on-site installation in fish farming. The efficiency of the filter gradually decreases. After a period the filter medium, will become saturated with nitrogen. Nitrogen gas will then follow the oxygen gas out of the generator and to the fish. For this reason two columns are always used in alternation: one is used and the other is regenerated. When a column is regenerated, air is ‘driven’ through the column in the opposite direction, and the nitrogen is blown out to the atmosphere.
An oxygen generator normally delivers oxygen gas with a maximum pressure of 4 bar. This must betaken into consideration because some of the equipment for injecting oxygen gas into the water may require higher pressure. If so, a specially designed oxygen compressor must be used after the oxygen generator. After the generator there must be a storage tank for the oxygen produced to ensure pressure equalization. From this tank the oxygen is tapped and used for injection into the water on the farm.
When a generator has been in use for some years (depending on type of equipment and running time) the purity of the delivered oxygen will fall to 80–85%. The amount of nitrogen gas will also increase, which can be toxic to the fish. If the oxygen gas is supplied under high pressure, there will be possibilities for supersaturation of nitrogen. Maintenance and replacement or cleaning of the filter medium inside the columns must then be carried out. It is therefore important to sample the oxygen gas from the generators at fixed intervals and measure its purity. An easy way to do this is to collect a sample of the oxygen gas in a plastic bag. The oxygen meter that is used to measure the content of oxygen in the water on the farm can then be used to measure the approximate oxygen purity. This is done by first adjusting the meter to show 21% purity in air; now when the oxygen probe is put into the plastic bag, it will show the purity
of the oxygen gas sample directly, for instance 95%.
Selection of source
The choice of oxygen source depends on several factors. If the oxygen consumption is very low or if oxygen is only used for security purposes, it is normal to use gas bottles. Otherwise there is a choice between purchasing liquid oxygen and on-site production. If only the price of oxygen is con-sidered, on-site production will in almost every case be cheaper. The reliability, maintenance costs and necessary back-up system, however, often cause farmers to buy liquid oxygen from commercial producers because they guarantee the oxygen supply. Other important factors when choosing are the distance to the oxygen production factory, infrastructure for transport of liquid oxygen, and the price of electricity. The amount of electric energy used for production of oxygen gas is size-dependent; larger generators are more efficient than smaller ones.
Related Topics