Chapter: Aquaculture Engineering : Aeration and Oxygenation
Gases in water
Gases in water
Water contains a certain amount of dissolved gases. When the
water has taken up the amount possible under normal atmospheric pressure it is
fully saturated (100%) or in equilibrium. If the water
contains less gas than can be taken up at a given temperature, it is less than
saturated (<100%); if the water contains more gas than when fully saturated,
it is super-saturated (>100%).
The percentage saturation can be calculated by dividing the
measured concentration (Cm)
by the concentration at saturation (Cs):
Percentage saturation = (Cm/Cs) × 100
All the gases in the atmosphere can be dissolved in water; the
sum of the partial pressures of all the dissolved gases is known as the total
gas pressure (TGP). The pressure difference P
is the difference between TGP in the water and the barometric pres-sure (BP) of
the air above the water:
∆P =TGP−BP
If TGP measured in the water is higher than the BP of the air
(positive P), the water is
super-saturated and gas will be forced out of the water. If TGP is less than
BP, the water is under saturated and gases will be forced into the water. TGP
may be expressed as a percentage of the BP:
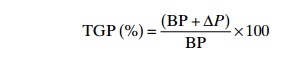
The water vapour pressure, PH2O,
may also be included in the equation:
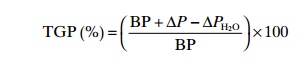
Air and water contains many gases, but in fish farming oxygen,
nitrogen, and in some cases also carbon dioxide, are of greatest importance.
Since the major reason for adding water is to provide oxygen, the added water
should have as high an oxygen concentration as possible, close to 100%
saturation. If under saturated water is used, more water must be supplied.
Water may also be super-saturated with oxygen, i.e. its concentration in the
water is raised above 100% by the addition of pure oxygen, to reduce the amount
of water that must be supplied to the fish. If the other gases in the water are
in equilibrium, water that is super-saturated with oxygen will have a positive P, i.e. TGP is higher than BP. The
concentration of nitrogen in the water must not be above 100%, because this can
cause bubble (diving) disease in the fish.
Water treatment and biological processes in the water source can
result in both super-saturation and under saturation of the different gases.
Super-saturation can result from naturally occurring or man-made processes.5
Super-saturation can be the result of rapid heating of water, mixing of water
of different temperatures, freezing of water when some of the gases remain in
the water, mixing air into the water, for instance under waterfalls or by
waves, photosynthesis that creates oxygen gas, and changes in the BP.
If the saturation of the gases is 100% or less, they are
completely dissolved in the water. However, if the concentration of gas is
above 100% saturation, there will be free gas bubbles in the water; these are
small and difficult to observe. Only the oxygen actu-ally dissolved in the
water is available to the fish: when the water is super-saturated with oxygen,
the bubbles will gradually be dissolved in the water and the oxygen made
available for the fish as the fish consume the oxygen that is already dissolved
in the water.
Air and water have different gas contents; in both nitrogen and
oxygen are the major gases. In air the relation between nitrogen and oxygen is
approximately 78%–21% by volume (Table 8.1), while in
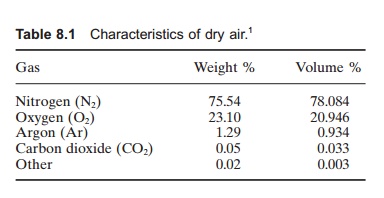
water the relation is 60% nitrogen and 40% oxygen when the gases
are in equilibrium. It is important to be aware of this relationship because if
air is pressed into the water, super-saturation of nitrogen may occur which is
harmful to the fish.
The air will exert pressure on the water surface. At sea level
this pressure is 1 atmosphere, equal to 760 ± 25 mmHg; it varies depending on
atmospheric conditions. The oxygen partial pressure is related to the
percentage of the total volume that oxygen constitutes, which in air is 21%;
multiplying this value by the BP (1 atm or 760 mmHg) gives the partial pressure
of oxygen.
Partial pressure O2= 0.21 × 760 mmHg = 159.6 mmHg
This means that the pressure of oxygen on the water surface is
159.6 mmHg.
The total pressure represented by the BP is the sum of the
partial pressures of the gases in the atmosphere. This can be described as follows,
neglecting the gases that constitute a very small proportion of the air:
BP =P(N2) +P(O2) +P(Ar) +P(CO2)
The amount of a gas dissolved in water is referred to as the
solubility of the gas, and depends on several factors, such as water
temperature, pressure, salinity and substances in the water.6 At
higher tem-perature gases are less soluble in water because the molecules then
need more space. The solubility of oxygen and nitrogen decreases linearly with
increasing temperature (see later) and for this reason hot water contains less
oxygen than cold water. The solubility for oxygen and nitrogen also decreases
with increased salt content of the water. Tables for the content of oxygen in
water with different temperatures and salinity is given in Appendix 8.1.
The solubility of gases in water is normally expressed as mg/l
of the actual gas, but it may also be expressed as the partial pressure.
Equations for conversion are available; for oxygen and carbon
dioxide the following may be used:

where:
partial pressure for the actual gas (i) is in mmHg
Ci=concentration of the actual gas in mg/l
βi=Bunsen coefficient for the actual gas, depending on temperature
and salinity (Appendix 8.2)
Ai=constant depending on the actual gas (0.5318for oxygen and
0.3845 for carbon dioxide).
Solubility may also be expressed on the basis of tension. The
oxygen tension may, for instance, be the necessary partial pressure in the
atmosphere to keep a certain concentration in the water. If the atmosphere is
air at normal pressure, the oxygen tension is 159 mmHg. This creates 100%
oxygen saturation in the water. If the pressure is less than this, the
concentration in the water will also be reduced.
Related Topics