Chapter: Aquaculture Engineering : Ammonia Removal
Ammonia Removal - Aquaculture Engineering
Ammonia Removal
Introduction
In aquaculture it is often
necessary to reduce the concentration of ammonia in the water, because it is
toxic for the fish. It is particularly
important when re-using water or when trans-porting fish long distances without
changing the water, since the fish produce ammonium com-pounds as a metabolic
waste product.
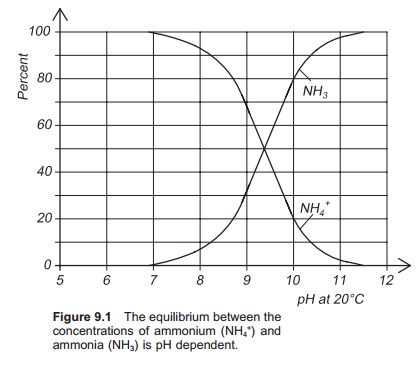
In water there is an equilibrium
between the concentrations of ammonium ion (NH4+) and ammonia (NH3) (Fig. 9.1):
NH3+ H+⇔ NH4+
This equilibrium depends on pH.
The sum of NH3 and NH4+ is known as the total ammonia nitrogen (TAN). Because of the
equilibrium between NH3 and NH4+, reducing one of them automatically reduces the other. NH3 is the more toxic substance for
fish, and therefore is the substance of interest.
Related Topics