Chapter: Aquaculture Engineering : Ammonia Removal
Nitrification
Nitrification
Nitrification
The nitrification process is carried out in two steps and is performed by bacteria which oxidize ammonia. These bacteria are autotrophic and use O2 as oxidizing agent and CO2 or HCO−3 as a carbon source for growth. NH4+ is transformed to NO−2 byNitrosomonas bacteria and then to NO−3 by Nitrobacter bacteria. Both of these processes require energy that is supplied by the substrate. The chemical processes involved are as follows:
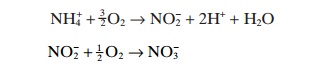
The bacteria grow in the biofilm on the filter medium. Nitrification takes place in this film so the biofilm must be established for the nitrification filter to function. The process creates more biofilm as the bacteria grow and divide, and the cell mass increases. This cell mass can be described as C5H7NO2. The processes including creation of cell mass can then be described as follows.
Step 1 using Nitrosomonas bacteria:
55NH4++ 5CO2+ 76O2→ C5H7NO2+ 54NO−2+ 109H+ + 52H2O
Step 2 using Nitrobacter bacteria:
400NO2+ 5CO2+ 76O2→ C5H7NO2+ 400NO−3+ H+
The effectiveness of a nitrification filter can be described by the nitrification rate, defined as the amount of ammonium oxidized per unit biofilm surface area and unit time (mg NH4+/(m2 min)). The efficiency of the nitrification process and the establishment of the biofilm process depend on several factors.11It is important that the bacteria grow as optimally as possible.
The following important factors regulates the growth of the bacterial culture:
· Concentration of ammonia
· Temperature
· Oxygen concentration
· pH
· Salinity
· Organic substances
· Toxic substances.
One of the main factors affecting bacterial growth is the amount of ammonia in the water. In fish farming this concentration is normally too low for maximal growth, compared to municipal waste-water, for instance, where the same processes is used. Generally values above 3 mg ammonium nitrogen per litre are recommended for maximal growth. Values that are too high may inhibit bacterial growth.
Since these bacterial cultures requires oxygen to grow, and in order to have transformation of ammonia to nitrate, it is important that sufficient oxygen is available throughout the entire nitrification process. Experiments have demonstrated a reduction in Nitrosomonasactivity with oxygen levels below 4 mg/l water, while the corresponding value for Nitrobacter is 2 mg/l.
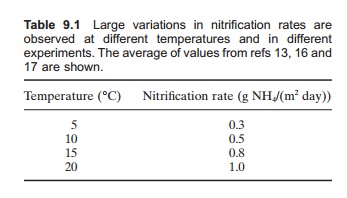
Bacterial growth rate depends on the tempera-ture.13–15 Bacterial activity occurs from 0 to 30°C and increases with temperature: the optimal range is around 30°C. Temperatures that are too high will result in mortality. However, the bacteria may accli-mate to lower temperatures over time, so high bac-terial activity can also be achieved at lower temperatures. If the temperature reaches values below 5°C growth will be slow and it will be difficult and time-consuming to establish new bacterial cultures. It is difficult to give general values for the nitrification rate in relation to temperature, due to both acclimation and the number of other factors affecting bacterial metabolism (Table 9.1).
Nitrification also depends on the pH of the water: optimal values are between 8 and 9.18 It was shown experimentally that the nitrification rate decreased by 90% when the pH was reduced from 7 to 6. It is important to remember that hydrogen ions (H+) are produced in the nitrification process and, depending on the buffering capacity of the water, may reduce the pH. If the loads on the biofilter are high, systems for adjusting the pH are necessary; addition of lime is an example.
The presence of organic matter can inhibit the functioning of the nitrification filter.19–21Other bacterial cultures may start to grow inside the filters in competition with nitrification bacteria. Heterotrophic aerobic bacteria may use the organic matter as a carbon source and replace the nitrification bacteria because they have a faster growth rate. Nitrification will be reduced by increasing the carbon/nitrogen ratio; a 60–70% reduction in nitrification rate was observed when increasing the chemical oxygen demand/nitrogen (COD/N) ratio from 0 to 3 for a substrate containing 10 mg TAN.
Some substances may be toxic to nitrification bacteria and may kill or inhibit the bacterial culture in the biofilter. These can include metal ions, organic substances or medicaments such as formaldehyde.
Salinity affects the nitrification rate because the chloride ions inhibit bacterial growth. For this reason the nitrification is faster in freshwater than in seawater.
Nitrification filters need to be shielded from light, because it may reduce nitrification. Predators may also have a negative influence.
The problem about giving reliable values for the nitrification rate is, of course, that all the factors affect each other. For this reason it is also normal to incorporate high safeguards when designing nitrification filters.
Related Topics