Chapter: 10th Science : Chapter 11 : Carbon and its Compounds
Soaps and Detergents
SOAPS AND DETERGENTS
Soaps and the Detergents
are materials that are used by us for cleaning purposes because pure water
alone cannot remove all types of dirt or any oily substance from our body or
clothes. They contain ‘surfactants’, which are compounds with molecules that
line up around water to break the ‘surface tension’. Both of them having a
different chemical nature. Soap is a cleaning agent that is
composed of one or more salts of fatty acids. Detergent is a
chemical compound or a mixture of chemical compounds, which is used as a
cleaning agent, also. They perform their cleaning actions in certain specific
conditions. You will learn more about this in detail, in the following units.
1. Soap
Soaps are sodium or
potassium salts of some long chain carboxylic acids, called fatty acids. Soap requires two major
raw materials: i) fat and ii) alkali. The alkali, most commonly used in the
preparation of soap is sodium hydroxide. Potassium hydroxide can also be used.
A potassium-based soap creates a more water- soluble product than a
sodium-based soap. Based on these features, there are two types of soaps:
A. HARD SOAP
Soaps, which are
prepared by the saponification of oils or fats with caustic soda (sodium
hydroxide), are known as hard soaps. They are usually used for washing
purposes.
B. SOFT SOAP
Soaps, which are
prepared by the saponification of oils or fats with potassium salts,
are known as soft soaps. They are used for cleansing the body.
Manufacture of soap
KETTLE PROCESS:
This is the oldest
method. But, it is still widely used in the small scale preparation of soap.
There are mainly, two steps to be followed in this process.
i) Saponification of oil:
The oil, which is used
in this process, is taken in an iron tank (kettle). The alkaline solution (10%)
is added into the kettle, a little in excess. The mixture is boiled by passing
steam through it. The oil gets hydrolysed after several hours of boiling. This
process is called Saponification
ii) Salting out of soap:
Common salt is then
added to the boiling mixture. Soap is finally precipitated in the tank. After
several hours the soap rises to the top of the liquid as a ‘curdy mass’. The
neat soap is taken off from the top. It is then allowed to cool down.
Effect of hard water on soap
Hard water contains
calcium and magnesium ions (Ca2+ and Mg2+) that limit the cleaning action of
soap. When combined with soap, hard water develops a thin layer (precipitates
of the metal ions) called ‘scum’, which leaves a deposit on the clothes or skin
and does not easily rinse away. Over time, this can lead to the deterioration
of the fabric and eventually ruin the clothes. On the other hand, detergents
are made with chemicals that are not affected by hard water.
2. Detergents
Development of synthetic
detergents is a big achievement in the field of cleansing.
These soaps possess the
desirable properties of ordinary soaps and also can be used with hard water and
in acidic solutions. These are salts of sulphonic acids or alkyl hydrogen
sulphates in comparison to soap, which are salts of carboxylic acids. The
detergents do not form precipitates with Ca2+ and Mg2+ present in hard water.
So, the cleansing action of detergents is better than that of soaps.
Preparation of detergents
Detergents are prepared
by adding sulphuric acid to the processed hydrocarbon obtained from petroleum.
This chemical reaction result in the formation of molecules similar to the
fatty acid in soap. Then, an alkali is added to the mixture to produce the
‘surfactant molecules’, which do not bond with the minerals present in the hard
water, thus preventing the formation of their precipitates.
In addition to a
‘surfactant’, the modern detergent contains several other ingredients. They are
listed as follows:
i) Sodium silicate,
which prevents the corrosion and ensures that the detergent does not damage the
washing machine.
ii) Fluorescent
whitening agents that give a glow to the clothes.
iii) Oxygen bleaches,
such as ‘sodium perborate’, enable the removal of certain stains from the
cloth.
iv) Sodium sulphate is
added to prevent the caking of the detergent powder.
v) Enzymes are added to
break down some stains caused by biological substances like blood and vegetable
juice.
vi) Certain chemicals
that give out a pleasant smell are also added to make the clothes fragrant
after they are washed with detergents.
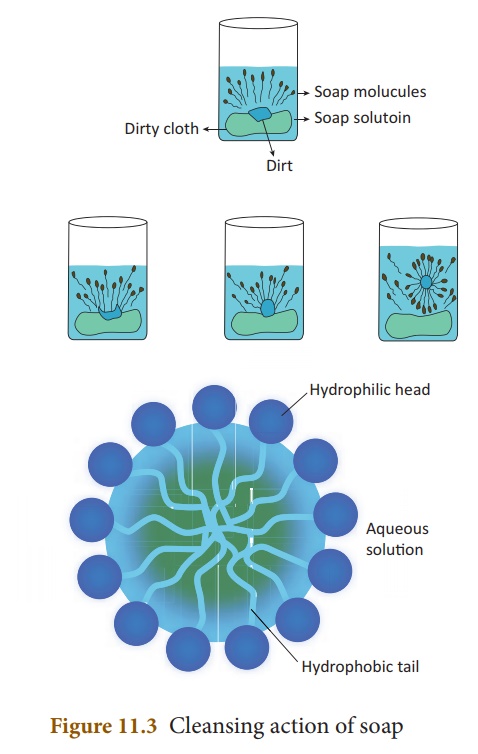
3. Cleansing action of soap
A soap molecule contains
two chemically distinct parts that interact differently with water. It has one polar
end, which is a short head with a carboxylate
group (–COONa) and one non-polar end having the long
tail made of the hydrocarbon chain.
The polar end is hydrophilic
(Water loving) in nature and this end is attracted towards
water. The non -polar end is hydrophobic (Water hating) in nature
and it is attracted towards dirt or oil on the cloth, but not
attracted towards water. Thus, the hydrophobic part of the soap molecule traps
the dirt and the hydrophilic part makes the entire molecule soluble in water.
When a soap or detergent
is dissolved in water, the molecules join together as clusters called ‘micelles’.
Their long hydrocarbon chains attach themselves to the oil and dirt. The dirt
is thus surrounded by the non-polar end of the soap molecules (Figure 11.3).
The charged carboxylate end of the soap molecules makes the micelles soluble in
water. Thus, the dirt is washed away with the soap.
Advantages of detergents over soaps
Detergents are better
than soaps because they:
·
can be used in both hard and soft water and can clean more
effectively in hard water than soap.
·
can also be used in saline and acidic water.
·
do not leave any soap scum on the tub or clothes.
·
dissolve freely even in cool water and rinse freely in hard water.
·
can be used for washing woollen garments, where as soap cannot be
used.
·
have a linear hydrocarbon chain, which is biodegradable.
·
are active emulsifiers of motor grease.
·
do an effective and safe cleansing, keeping even synthetic fabrics brighter and
whiter.
Biodegradable and Non-biodegradable detergents:
a) Biodegradable detergents:
They have straight
hydrocarbon chains, which can be easily degraded by bacteria.
b) Non-biodegradable detergents:
They have highly
branched hydrocarbon chains, which cannot be degraded by bacteria.
Disadvantages of
Detergents
1. Some detergents
having a branched hydro-carbon chain are not fully biodegradable by
micro-organisms present in water. So, they cause water pollution.
2. They are relatively more expensive than soap.
4. Comparison between soap and detergents
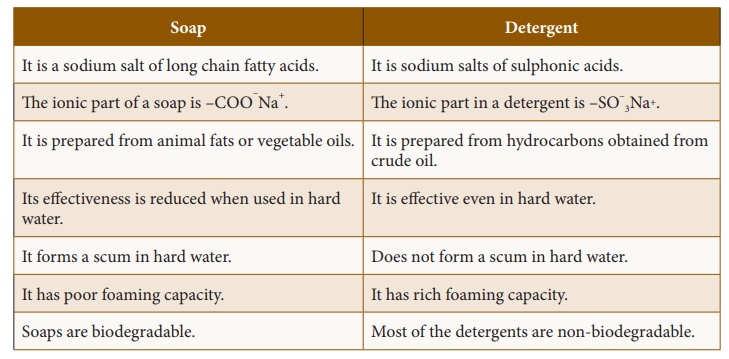
Soap
·
It is a sodium salt of long chain fatty acids.
·
The ionic part of a soap is –COO–Na+
·
It is prepared from animal fats or vegetable oils.
·
Its eff ectiveness is reduced when used in hard water.
·
It forms a scum in hard water.
·
It has poor foaming capacity.
·
Soaps are biodegradable.
Detergent
·
It is sodium salts of sulphonic acids.
·
The ionic part in a detergent is –SO–3Na+.
·
It is prepared from hydrocarbons obtained from crude oil.
·
It is effective even in hard water.
·
Does not form a scum in hard water.
·
It has rich foaming capacity.
·
Most of the detergents are non-biodegradable.
Related Topics