Manufacture, Physical and Chemical Properties, Uses - Ethanol | 10th Science : Chapter 11 : Carbon and its Compounds
Chapter: 10th Science : Chapter 11 : Carbon and its Compounds
Ethanol
ETHANOL (CH3CH2OH)
Ethanol is commonly
known as alcohol. All alcoholic beverages and some cough syrups contain
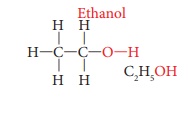
ethanol. Its molecular formula is C2H5OH. Its structural
formula is
1. Manufacture of ethanol
Ethanol is manufactured
in industries by the fermentation of molasses, which is a by -product obtained
during the manufacture of sugar from sugarcane. Molasses is a dark coloured
syrupy liquid left after the crystallization of sugar from the concentrated
sugarcane juice. Molasses contain about 30% of sucrose, which cannot be separated
by crystallization. It is converted into ethanol by the following steps:
(i) Dilution of molasses
Molasses is first
diluted with water to bring down the concentration of sugar to about 8 to 10
percent.
(ii) Addition of Nitrogen source
Molasses usually contains
enough nitrogenous matter to act as food for yeast during the fermentation
process. If the nitrogen content of the molasses is poor, it may be fortified
by the addition of ammonium sulphate or ammonium phosphate.
(iii) Addition of Yeast
The solution obtained in
step (ii) is collected in large ‘fermentation tanks’ and yeast is added to it.
The mixture is kept at about 303K for a few days. During this period, the
enzymes invertase and zymase present in yeast, bring about the conversion of
sucrose into ethanol.
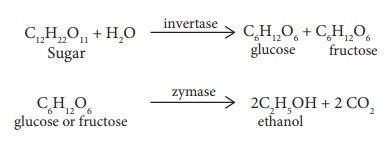
The fermented liquid is
technically called wash.
(iv) Distillation of 'Wash'
The fermented liquid
(i.e. wash), containing 15 to 18 percent alcohol, is now subjected to
fractional distillation. The main fraction drawn is an aqueous solution of
ethanol which contains 95.5% of ethanol and 4.5% of water. This is called rectified
spirit. This mixture is then refluxed over quicklime for about 5 to
6 hours and then allowed to stand for 12 hours. On distillation of this
mixture, pure alcohol (100%) is obtained. This is called absolute alcohol.
2. Physical properties
i) Ethanol is a colourless liquid, having a pleasant smell and a
burning taste.
ii) It is a volatile liquid. Its boiling point is 780 C (351K),
which is much higher than that of its corresponding alkane, i.e. ethane
(Boiling Point = 184 K).
iii) It is completely miscible with water in all proportions.
3. Chemical Properties
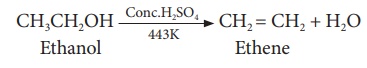
(i) Dehydration (Loss of water)
When ethanol is heated
with con H2SO4 at 443K, it loses a water molecule i.e.
dehydrated to form ethene.
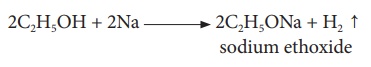
(ii) Reaction with sodium:
Ethanol reacts with
sodium metal to form sodium ethoxide and hydrogen gas.
2C2H5OH
+ 2Na → 2C2H5ONa + H2 ↑ sodium ethoxide
(iii) Oxidation:
Ethanol is oxidized to
ethanoic acid with alkaline KMnO4 or acidified K2Cr2O7
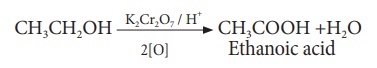
During this reaction,
the orange colour of K2Cr2O7 changes to green.
Therefore, this reaction can be used for the identification of alcohols.
(iv) Esterification:
The reaction of an
alcohol with a carboxylic acid gives a compound having fruity odour. This
compound is called an ester and the reaction is called esterification.
Ethanol reacts with ethanoic acid in the presence of conc. H2SO4
to form ethyl ethanoate, an ester.
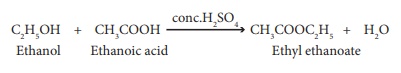
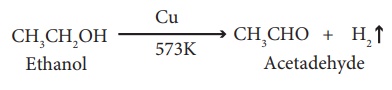
(v) Dehydrogenation:
When the vapour of
ethanol is passed over heated copper, used as a catalyst at 573 K, it is
dehydrogenated to acetaldehyde.
(vi) Combustion:
Ethanol is highly
inflammable liquid. It burns with oxygen to form carbon dioxide and water
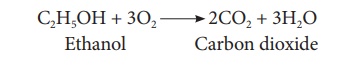
4. Uses of ethanol
Ethanol is used
� in medical wipes, as an
antiseptic.
� as an anti-freeze in
automobile radiators.
� for effectively killing
micro organisms like bacteria, fungi, etc., by including it in many hand
sanitizers.
� as an antiseptic to
sterilize wounds in hospitals.
� as a solvent for drugs,
oils, fats, perfumes, dyes, etc.
� in the preparation of methylated
spirit (mixture of 95% of ethanol and 5% of methanol) rectified spirit
(mixture of 95.5% of ethanol and 4.5% of water), power alcohol (mixture of
petrol and ethanol) and denatured spirit (ethanol mixed with pyridine).
� to enhance the flavour
of food extracts, for example vanilla extract; a common food flavour, which is
made by processing vanilla beans in a solution of ethanol and water.
Related Topics