Chapter: 10th Science : Chapter 11 : Carbon and its Compounds
Nomenclature of Organic Compounds
NOMENCLATURE OF ORGANIC
COMPOUNDS
1. Why do we need nomenclature?
In ancient days, the
names of organic compounds were related to the natural things from which they
were obtained. For example, the formic acid was initially obtained by
distillation of ‘red ants’. Latin name of the red ant is ‘Formica’. So, the
name of the formic acid was derived from the Latin name of its source Later,
the organic compounds were synthesized from sources other than the natural
sources. So scientists framed a systematic method for naming the organic
compounds based on their structures. Hence, a set of rules was formulated by
IUPAC (International Union of Pure and Applied Chemistry) for the
nomenclature of chemical compounds.
2. Components of an IUPAC name
The IUPAC name of the
any organic compound consists of three parts:
i. Root word
ii. Prefix
iii. Suffix
These parts are combined
as per the following sequence to get the IUPAC name of the compound:

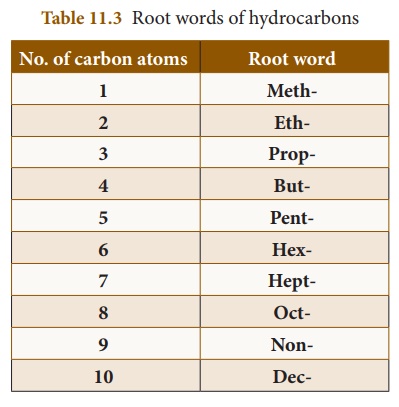
(i) Root word: It is the basic unit,
which describes the carbon skeleton. It gives the number of carbon atoms
present in the parent chain of the compound and the pattern of their
arrangement. Based on the number of carbon atoms present in the carbon
skeleton, most of the names are derived from Greek numerals (except the first
four). Table 11.3 shows the root words for the parent chain of hydrocarbons
containing 1to10 carbon atoms:
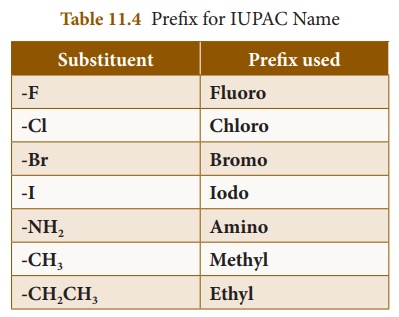
(ii) Prefix: The prefix represents
the substituents or branch present in the parent chain. Atoms or group
of atoms, other than hydrogen, attached to carbon of the parent chain are
called substituents. Table 11.4 presents the major substituents of organic
compounds and respective prefix used for them:
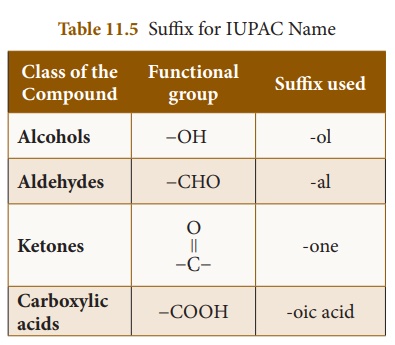
(iii) Suffix
The suffix forms the end
of the name. It is divided into two parts such as (a) Primary suffix and
(b) Secondary suffix. The primary suffix comes after the root word. It
represents the nature in carbon to carbon bonding of the parent chain.
If all the bonds between the carbon atoms of the parent chain are
single, then suffix ‘ane’ has to be used. Suffix ‘ene’ and ‘yne’
are used for the compounds containing double and triple bonds respectively. The
secondary suffix describes the functional group of the compound.
3. IUPAC rules for naming organic compounds:
� Rule1: Identify the longest
chain of carbon atoms to get the parent name (root word).
� Rule 2: Number the carbon atoms
of the parent chain, beginning at the closest end of the
substituent or functional group. These are called locant numbers.
If both functional group and substituent are present, then the priority
will be given to the functional group.
� Rule 3: In case of alkenes and
alkynes, locate the double bond or triple bond and use its locant number
followed by a dash and a primary suffix. The carbon chain is numbered in such a
way that the multiple bonds have the lowest possible locant number.
� Rule 4: If the compound contains
functional group, locate it and use its locant number followed by a dash
and a secondary suffix.
� Rule 5: When the primary and
secondary suffixes are joined, the terminal ‘e’ of the primary suffix is
removed.
� Rule 6: Identify the substituent and use a number followed by a dash and a prefix to specify its location and identity.
4. IUPAC Nomenclature of hydrocarbons – Solved examples
Let us try to name,
systematically, some of the linear and substituted hydrocarbons by following
IUPAC rules:
Example 1: CH3-CH2-CH2-CH2-CH3
Step 1: It is a five-
carbon chain and hence the root word is ‘Pent’. (Rule 1)
Step 2: All the bonds
between carbon atoms are single bonds, and thus the suffix is ‘ane’.
So, its name is Pent
+ ane = Pentane
Example 2:
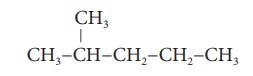
Step 1: The longest chain
contains five carbon atoms and hence the root word is ‘Pent’.
Step 2: There is a substituent.
So, the carbon chain is numbered from the left end, which is closest to
the substituent. (Rule 2)
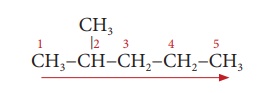
Step 2: All are single bonds
between the carbon atoms and thus the suffix is ‘ane’.
Step 3: The substituent is a
methyl group and it is located at second carbon atom. So, its locant
number is 2. Thus the prefix is ‘2-Methyl’. (Rule 6).
The name of the compound
is
2-Methyl + pent +ane =
2-Methylpentane
Example 3:
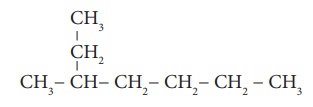
Step 1: The longest chain
contains seven carbon atoms and hence the root word is ‘Hept’.
Step 2: There is a substituent.
So, the carbon chain is numbered from the end, which is closest to substituent.
(Rule 2)
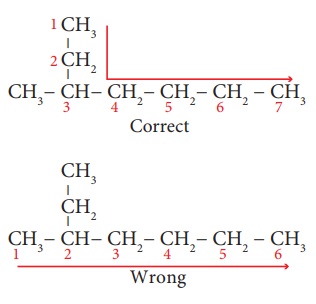
Step 2: All are single bonds
between the carbon atoms and thus the suffix is ‘ane’.
Step 3: The substituent is a
methyl group and it is located at third carbon. So, its locant number is
3. Thus the prefix is ‘3-Methyl’. (Rule 6)
Hence the name of the
compound is 3-Methyl + hept + ane = 3 –Methylheptane
Example 4: CH3-CH2-CH2-CH=CH2
Step 1: It is a ‘five- carbon
atoms chain’ and hence the root word is ‘Pent’. (Rule 1)
Step 2: There is a carbon to
carbon double bond. The suffix is ‘ene’.
Step 3: The carbon chain is
numbered from the end such that double bond has the lowest locant number
as shown below: (Rule 3):
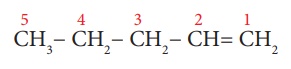
Step 4: The locant number of the
double bond is 1 and thus the suffix is ‘-1-ene’.
So, the name of the
compound is Pent + (-1-ene) = Pent-1-ene
5. IUPAC Nomenclature of other
classes – Solved examples





















Example 1: CH3-CH2-CH2-OH
Step1: The parent chain
consists of 3 carbon atoms. The root word is ‘Prop’.
Step 2: There are single bonds
between the carbon atoms of the chain. So, the primary suffix is
‘ane’.
Step 3: Since, the compound
contains – OH group, it is an alcohol. The carbon chain is numbered from
the end which is closest to –OH group. (Rule 3)
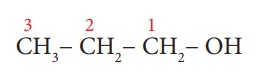
Step 4: The locant number of –OH
group is 1 and thus the secondary suffix is ‘1-ol’.
The name of the compound
is Prop + ane + (1-ol) = Propan-1-ol
Note: Terminal ‘e’ of ‘ane’ is removed as
per Rule 5
Example 2: CH3COOH
Step1: The parent chain
consists of 2 carbon atoms. The root word is ‘Eth’.
Step 2: All are single bonds
between the carbon atoms of the chain. So the primary suffix is ‘ane’.
Step 3: Since the compound
contains the–COOH group, it is a carboxylic acid. The secondary suffix
is ‘oic acid’
The name of the compound
is Eth + ane + oic acid) = Ethanoic acid
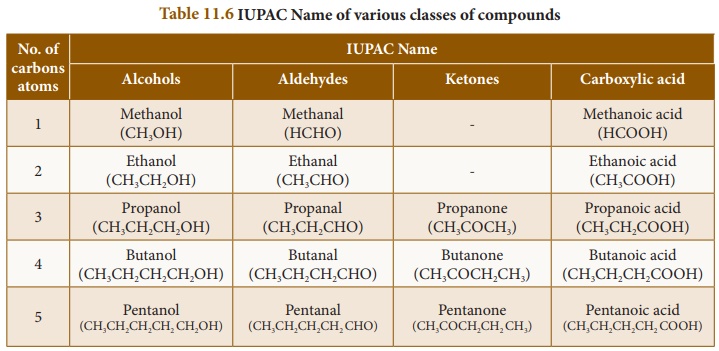
Table 11.6 lists IUPAC
names homologs of various classes of organic compounds
Test yourself:
Obtain the IUPAC name of
the following compounds systematically:
(a) CH3CHO
(b) CH3CH3COCH3
(c) ClCH2-CH2-CH2-CH3
Related Topics