Manufacture, Physical and Chemical Properties, Uses - Ethanoic acid | 10th Science : Chapter 11 : Carbon and its Compounds
Chapter: 10th Science : Chapter 11 : Carbon and its Compounds
Ethanoic acid
ETHANOIC ACID (CH3COOH)
Ethanoic acid or acetic
acid is one of the most important members of the carboxylic acid family. Its
molecular formula is C2H4O2. Its structural
formula is
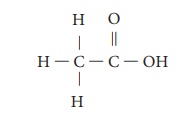
1. Manufacture of ethanoic acid
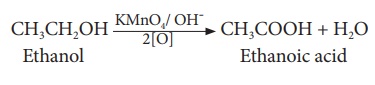
Ethanoic acid is
prepared in large scale, by the oxidation of ethanol in the presence of
alkaline potassium permanganate or acidified potassium dichromate.
2. Physical Properties
i.
Ethanoic acid is a colourless liquid having an unpleasant odour.
ii.
It is sour in taste.
iii.
It is miscible with water in all proportions.
iv.
Its boiling point is higher than the corresponding alcohols,
aldehydes and ketones.
v.
On cooling, pure ethanoic acid is frozen to form ice like flakes.
They look like glaciers, so it is called glacial acetic acid.
3. Chemical Properties
(i) Reaction with metal:
Ethanoic acid reacts with
active metals like Na, Zn, etc., to liberate hydrogen and form sodium
ethanoate.
2CH3COOH + Zn
→ (CH3COO)2 Zn + H2 ↑
2CH3COOH +
2Na → 2CH3COONa + H2 ↑
(ii) Reaction with
carbonates and bicarbonates: Ethanoic acid reacts with sodium
carbonate and sodium bicarbonate, which are weaker bases and liberates CO2,
with brisk effervescence.
2CH3COOH + Na2CO3
→ 2CH3COONa + CO2↑
+ H2O
CH3COOH +
NaHCO3 → CH3COONa + CO2↑ + H2O
(iii) Reaction with base: Ethanoic acid reacts with sodium
hydroxide to form sodium ethanoate and water.
CH3COOH +
NaOH → CH3COONa + H2O
(iv) Decarboxylation
(Removal of CO2): When a sodium salt of ethanoic acid is heated
with soda lime (solid mixure of 3 parts of NaOH and 1 part of CaO), methane gas
is formed.
CH3COONa→ NaOH
/ CaO →CH4 ↑
+ Na2CO3
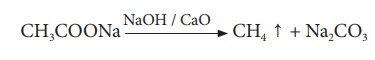
4. Uses of ethanoic acid
Acetic acid, in lower
concentration, is used as a food additive, a flavoring agent and a
preservative.
Ethanoic acid is used
� in the manufacture of
plastic.
� in making dyes,
pigments and paint.
� in printing on fabrics.
� as a laboratory
reagent.
� for coagulating rubber
from latex.
� in the production of
pharmaceuticals.
Related Topics