Chapter: Plant Biochemistry: Phloem transport distributes photoassimilates to the various sites of consumption and storage
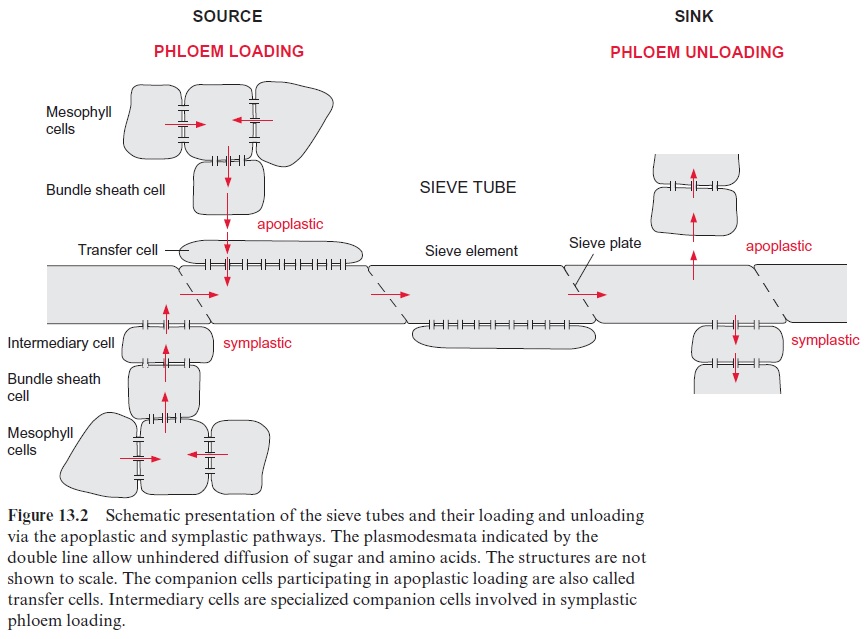
Sink tissues are supplied by phloem unloading
Sink tissues are supplied by phloem unloading
The delivered photosynthate is utilized in the sink tissues to sustain the metabolism, but may also be deposited there as reserves, mainly in the form of starch. There are again two possibilities for phloem unloading (Fig. 13.2). In symplastic unloading, the sucrose and amino acids reach the cells of the sink organs directly from the sieve elements via plasmodesmata. In apoplastic unloading, the compounds are first transported from the sieve tubes to the extracellular compartment and are then taken up into the cells of the sink organs. Electron microscopic investigations of the frequency of plasmodesmatal appearance indicate that in vegetative tissues, such as roots or growing shoots, phloem unloading proceeds primarily symplastically, whereas in storage tissues unloading is often, but not always, apoplastic.
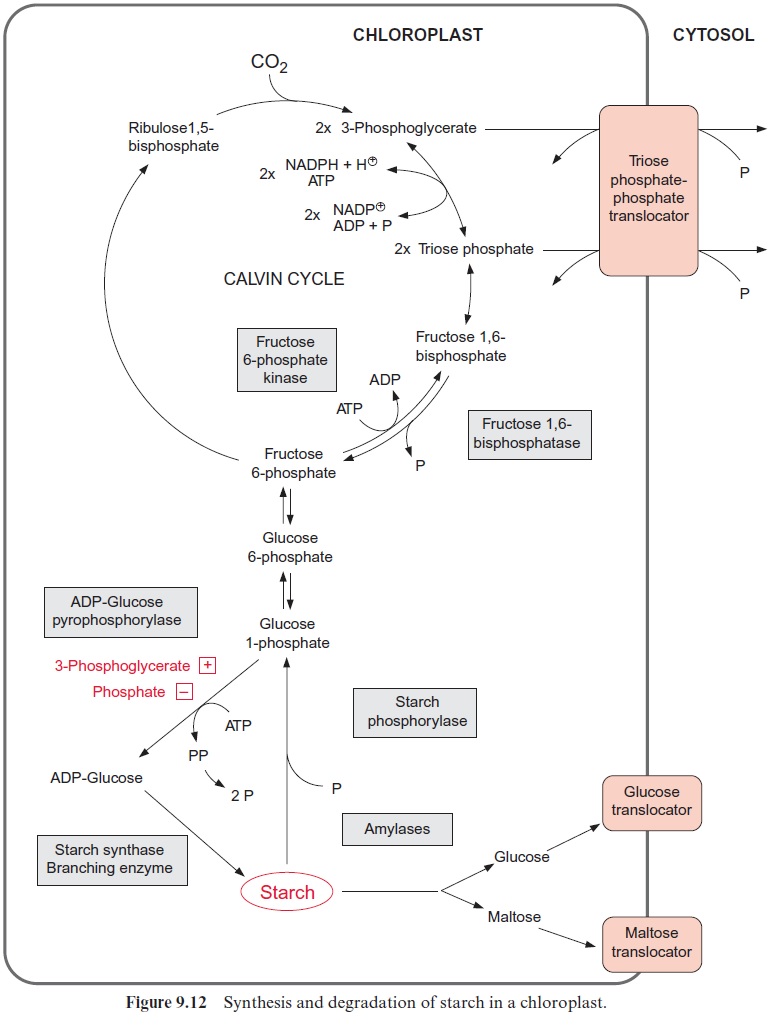
Starch is deposited in plastids
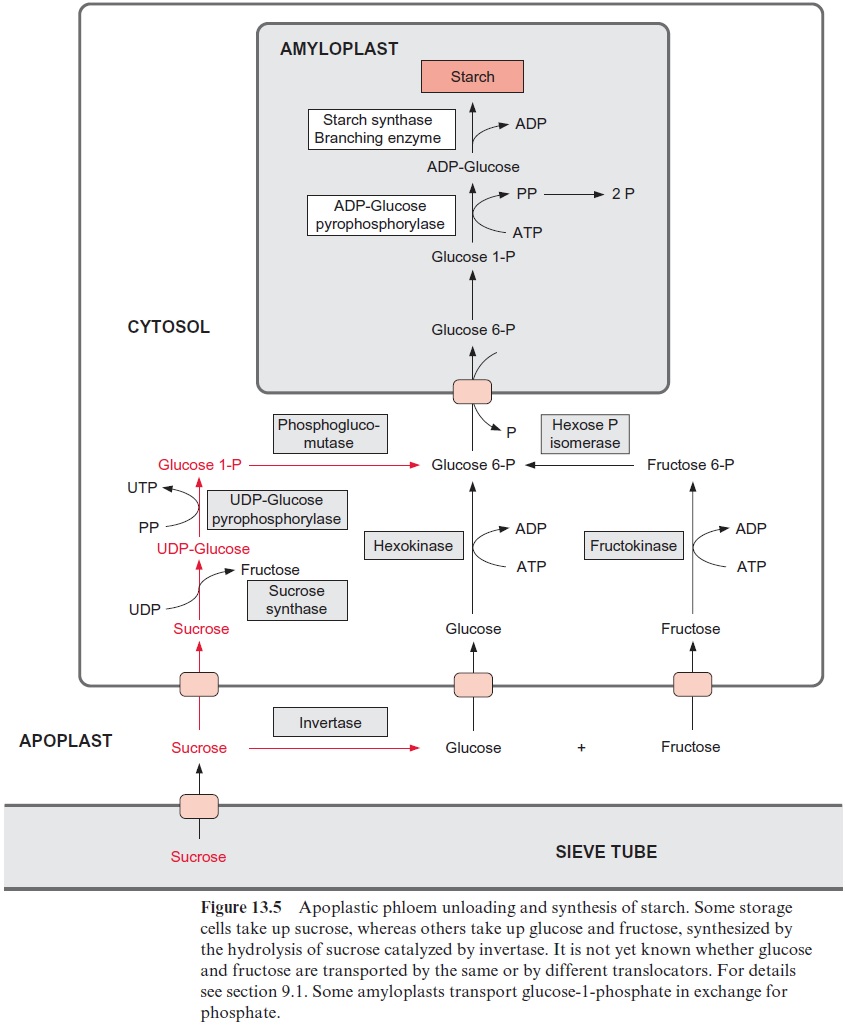
In storage tissues, the delivered carbohydrates are mostly converted to starch and stored as such. In apoplastic phloem unloading, this may pro-ceed by two alternative pathways. In the pathway colored red in Figure 13.5, the sucrose is taken up from the apoplast into the storage cells and converted there via sucrose synthase and UDP-glucose-pyrophosphory-lase to fructose and glucose 1-phosphate. In this reaction, pyrophosphate is consumed and UTP is generated. Phosphoglucomutase converts glucose 1-phosphate to glucose 6-phosphate. Alternatively, the enzyme invertase first hydrolyzes sucrose in the apoplast to glucose and fructose, and these two hexoses are then transported into the cell. This pathway is colored black in Figure 13.5. A fructokinase and a hexokinase (the latter phosphorylating mannose as well as glucose) catalyze the formation of the corresponding hexose phosphates. Glucose 6-phosphate is transported via the glucose 6-phosphate-phosphate translocator in counter-exchange for phosphate to the amyloplast, where starch is formed via the synthesis of ADP-glucose . Some leucoplasts transport glucose 1-phosphate in counter-exchange for phosphate. In potato tubers, the storage of starch probably proceeds mainly via sucrose synthase. In the taproots of sugar beet, the carbohydrates are stored as sucrose in the vacuoles. In some fruits (e.g., grapes), carbohydrates are stored in the vacuole as glucose.
The glycolysis pathway plays a central role in the utilization of carbohydrates
The carbohydrates delivered by phloem transport to the sink cells are fuel for the energy metabolism and also a carbon source for the synthesis of the cell matter. Theglycolysis pathway, which is present at least in part in almost all living organisms, has a fundamental role in the utilization of carbohydrates. The enzymes of this pathway not only occur in sink tissues but are also present in all plant cells. Each cell has two sets of glycolytic enzymes, one in the cytosol and one in the plastids. Some of the plastidic enzymes participate in the Calvin cycle. In the plastids of some plants, the glycolysis pathway is incomplete because one or two enzymes are lacking. The corresponding glycolytic enzymes in the cytosol and in the plastids are isoenzymes encoded by different genes.
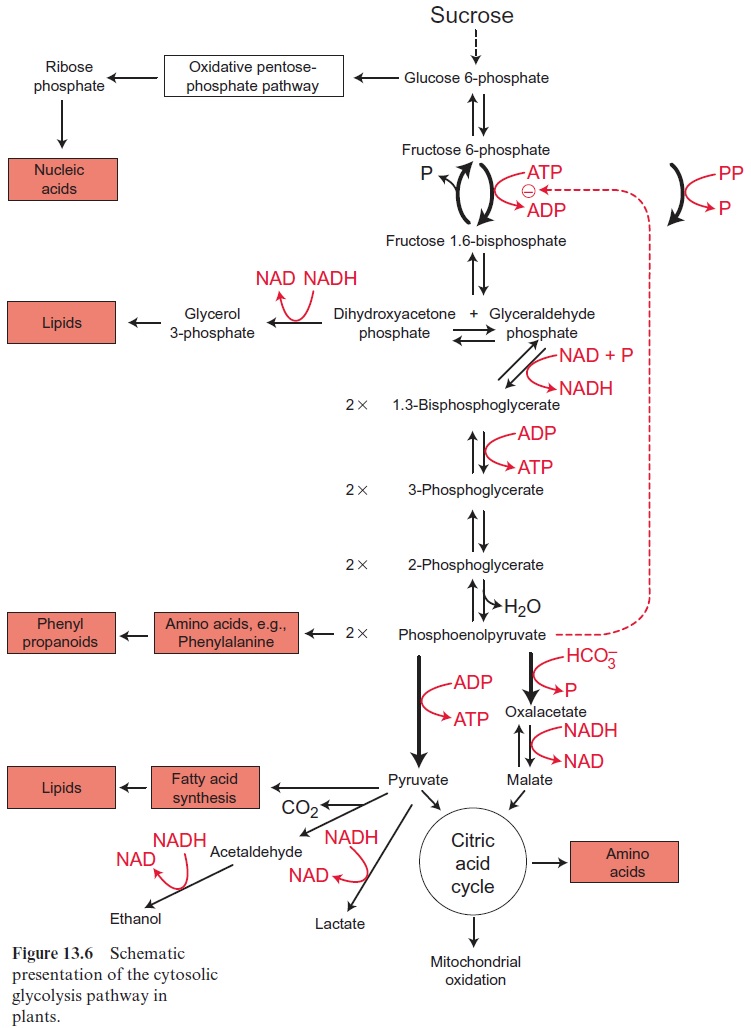
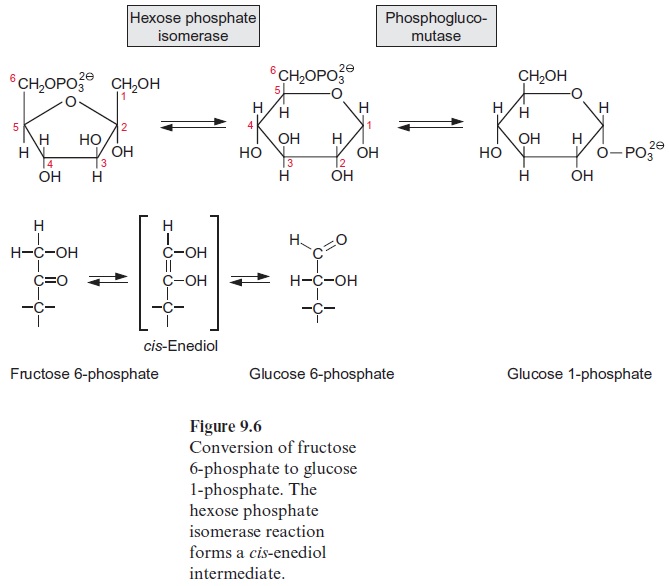
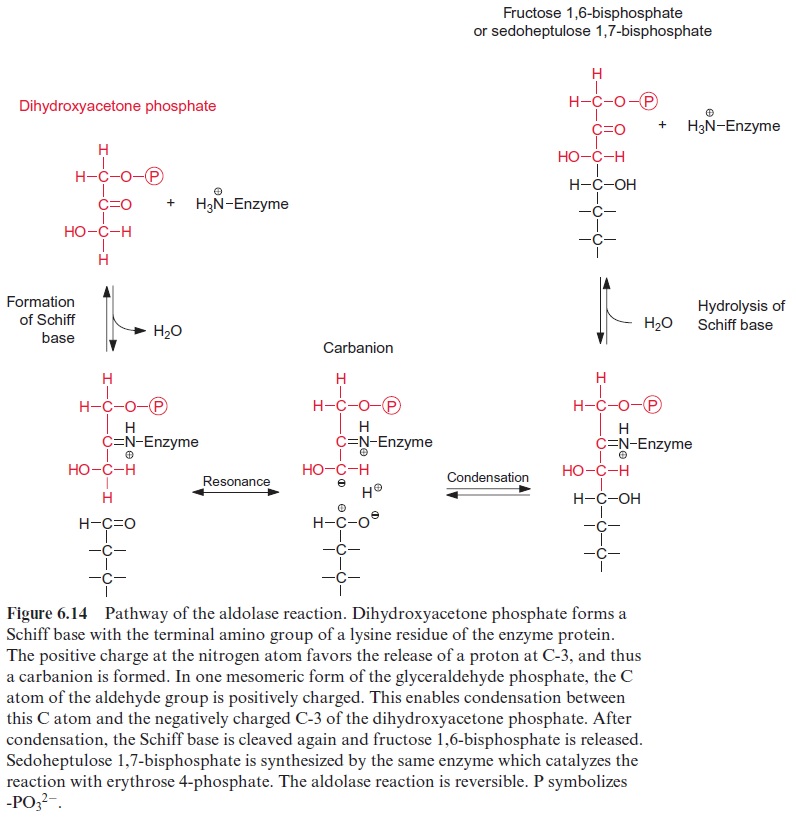
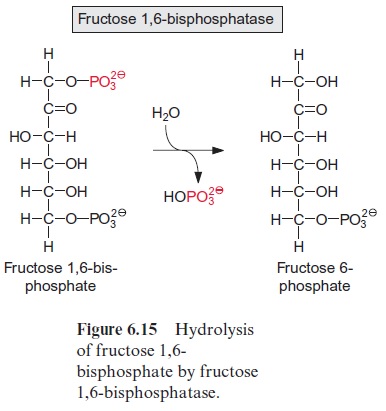
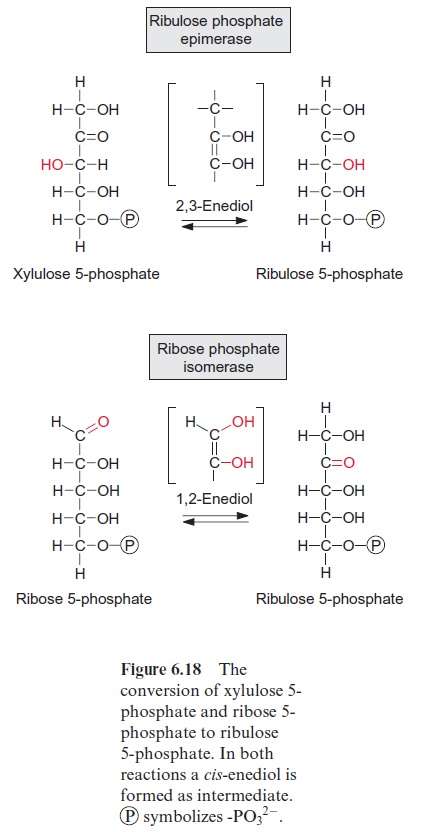
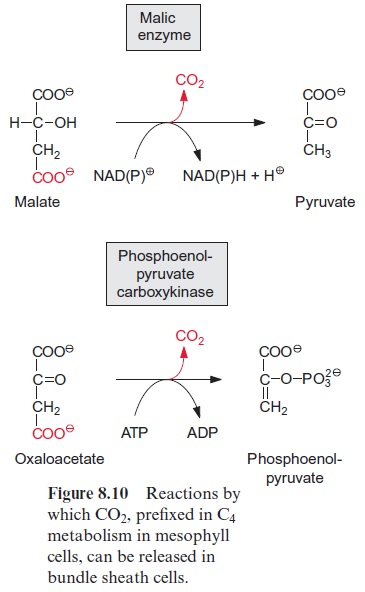
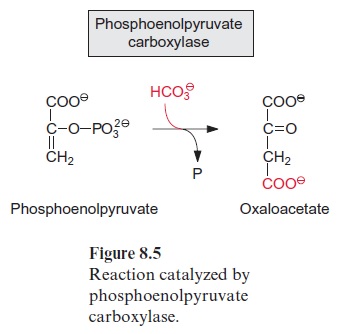
Figure 13.6 depicts the glycolysis pathway present in the cytosol. Glucose 6-phosphate, deriving from either the degradation of sucrose (Fig. 13.5) or the degradation of starch (Fig. 9.12), is converted in a reversible reaction by hexose phosphate isomerase to fructose 6-phosphate. This reaction proceeds in analogy to the isomerization of ribose 5-phosphate (Fig. 6.18). Fructose 6-phosphate is phosphorylated by ATP to fructose 1.6-bisphosphate, as catalyzed by ATP-phosphofructokinase (Fig. 9.15). Alternatively, it is also phosphorylated by inorganic pyrophosphate via pyrophosphate-phosphofructokinase. The latter enzyme does not occur in plastids. Since in both reactions the free energy for the hydrolysis of the anhydride phosphate donor is much higher than that of the phosphate ester, the formation of fructose 1.6-bisphosphate is an irreversible proc-ess. For this reason, the conversion of fructose 1.6-bisphosphate to fruc-tose 6-phosphate proceeds via another reaction, namely, the hydrolysis of phosphate, as catalyzed by fructose 1.6-bisphosphatase (Fig. 6.15). Fructose 1.6-bisphosphate is split in a reversible reaction into glyceraldehyde phos-phate and dihydroxyacetone phosphate as catalyzed by aldolase (Fig. 6.14). Dihydroxyacetone phosphate is converted to glyceraldehyde phosphate by triose phosphate isomerase, again in analogy to the isomerization of ribose 5-phosphate (Fig. 6.18). In the reaction sequence of the glycolysis pathway discussed so far, the hexose phosphate was prepared for the generation of reducing equivalents and of ATP. Glyceraldehyde phosphate is oxidized in a reversible reaction by glyceraldehyde phosphate dehydrogenase to 1.3-bisphosphoglycerate yielding the reduction of NAD+ . This reaction has already been discussed, although in the opposite direction, as part of the Calvin cycle in Figures 6.9 and 6.10. The change in free energy during the oxidation of the aldehyde to a carboxylate is conserved to form a phosphate anhydride, and by the reversible conversion of 1.3-bisphosphoglycerate to 3-phosphoglycerate, as catalyzed by phosphoglycerate kinase, this is uti-lized for the synthesis of ATP (Fig. 6.9). In order to prepare the remain-ing phosphate group for the synthesis of ATP, 3-phosphoglycerate is first converted by phosphoglycerate mutase to 2-phosphoglycerate (in analogy to the phosphogluco mutase reaction, Fig. 9.6) and then H2O is split off in a reversible reaction catalyzed by enolase, yielding phosphoenol pyruvate. In this way a phosphate ester is converted to an enol ester, of which the free energy of hydrolysis is considerably higher than that of the anhydride bond of ATP. Therefore the subsequent conversion of phosphoenolpyruvate to pyruvate coupled to the phosphorylation ADP by pyruvate kinase is an irreversible reaction. Alternatively, phosphoenolpyruvate can be converted in the cytosol via PEP carboxylase (Fig. 8.5) to oxaloacetate, and the latter can be reduced by malate dehydrogenase (Fig. 5.9) to malate. Malate can be converted to pyruvate by malic enzyme, as described in Figure 8.10. Both pyruvate and malate can be fed into the citrate cycle of the mitochondria for the generation of ATP via the respiratory chain
Under usual aerobic conditions, the glycolysis pathway makes only a minor contribution to the energy demand of a cell. The conversion of glu-cose 6-phosphate to pyruvate produces just three molecules of ATP. In contrast, mitochondrial oxidation of pyruvate and of the NADH formed by glyceraldehyde phosphate dehydrogenase yields about 25 molecules of ATP per glucose 6-phosphate. But in the absence of oxygen, which may occur when roots are flooded or during imbibition of water by ger-minating seeds, the ATP production by the glycolysis pathway is crucial for maintaining a minimal metabolism. In such a situation, the NADH generated in the glycolysis pathway can be reoxidized by the reduction of pyruvate to lactate, as catalyzed by lactate dehydrogenase. In roots and developing seeds, this enzyme is induced by a shortage of oxygen. The lactate formed is excreted as lactic acid. Alternatively, the NADH pro-duced by glycolysis can be consumed in converting pyruvate to ethanol, in the same way as in ethanol fermentation by yeast. In this reaction, pyru-vate is first decarboxylated by pyruvate decarboxylase to acetaldehyde, involving thiamine pyrophosphate as cofactor, similar as in Figure 5.4. Subsequently, alcohol dehydrogenase catalyzes the reduction of acetal-dehyde to ethanol, which is excreted. In plant cells, the activity of alco-hol dehydrogenase is largely increased as a response to oxygen deficit. In most plants, ethanol is the main product of anaerobic metabolism, with smaller amounts of lactic acid synthesized.
Apart from the generation of ATP, the glycolysis pathway provides precursors for a multitude of cell components. Here are a few examples: the oxidation of glucose 6-phosphate by the oxidative pentose phosphate path-way (Fig. 6.21) yields ribose 5-phosphate as a precursor for the synthesis of nucleotides and nucleic acids. The reduction of dihydroxyacetone phos-phate by glycerol phosphate dehydrogenase yields glycerol 3-phosphate, a precursor for the synthesis of lipids. Phosphoenolpyruvate is the precursor of a number of amino acids (e.g., phenylalanine), which is the precursor for the synthesis of phenylpropanoids, such as lignin (Fig. 18.9) and tannin (Fig. 18.16). Pyruvate is the precursor for the synthesis of fatty acids (Fig. 15.7) and hence the synthesis of lipids.
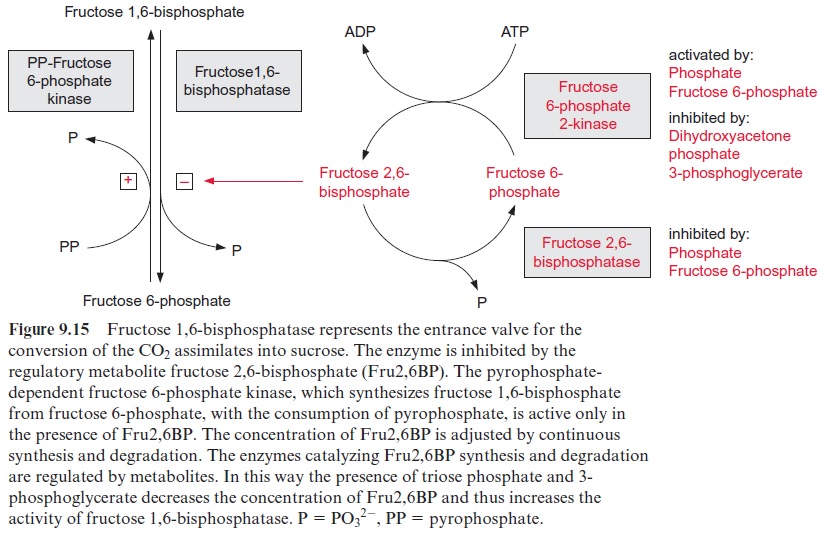
The glycolysis pathway is governed by a very complex regulation, particularly as most of the enzymes are located in the cytosol as well as in the plastids. In both compartments, the phosphorylation of fructose 6-phosphate by ATP-phosphofructokinase is inhibited by phosphoe-nolpyruvate, allowing a feedback control of the glycolysis pathway. The phosphorylation by the pyrophosphate-dependent phosphofructokinase is activated by fructose 2.6-bisphosphate, whereas the hydrolysis of fructose 1.6-bisphosphate by fructosebisphosphatase is inhibited by this compound (see Fig. 9.15).
Related Topics