Chapter: Modern Medical Toxicology: Neurotoxic Poisons: Drugs Used in Psychiatry
Selective Serotonin Reuptake Inhibitors (SSRI) - Antidepressants
Selective Serotonin Reuptake Inhibitors (SSRI)
These
drugs constitute the second generation of antidepressant drugs and are much
safer and better tolerated than the first generation drugs (cyclics and monoamine-oxidase inhibitors). Important examples include
citalopram, duloxetine, escit-alopram, fluoxetine, fluvoxamine, milnacipran,
oxaflozane, paroxetine, pizotifen, sertraline, venlafaxine. A related group of
drugs comprises the selective
serotonin-noradrenaline reup-take inhibitors (SNRIs), mainly represented by venlafaxine,milnacipram, and
duloxetine. For the sake of convenience, both groups are discussed together
under one heading.
Uses
Treatment
of
Ō¢ĀŌ¢Ā Depression
Ō¢ĀŌ¢Ā Panic
disorder
Ō¢ĀŌ¢Ā Obsessive-compulsive
disorder
Ō¢ĀŌ¢Ā Sleep disorders
Ō¢ĀŌ¢Ā Migraine
Ō¢ĀŌ¢Ā Substance
abuse.
Toxicokinetics
┬Ę
All SSRIs (except paroxetine) are
rapidly absorbed on oral administration. Because of slower absorption in the
case of paroxetine, symptoms of toxicity can be delayed. Peak plasma
concentrations are generally reached in about 2 to |8 hours depending on the
drug. Sertraline is also slowly absorbed; peak plasma concentrations are
reached approxi- mately 5 to 8 hours after oral dosing.
┬Ę
Protein binding ranges from 50% (for
citalopam) to 99% (for sertraline). Fluoxetine binds to plasma proteins to the
extent of 94%.
┬Ę
The primary route of elimination of
most of these drugs appears to be renal. Elimination half-lives range from 15
to 26 hours.
Mode of Action
The
SSRIs specifically inhibit the reuptake of serotonin, thereby potentiating the
activity of neuronally released serotonin.
Ō¢ĀŌ¢ĀThey also alter the sensitivity of serotonin subtype 5HT1A
or 5HT1C receptors.
Ō¢ĀŌ¢Ā Sertraline has a
greater selectivity for inhibiting 5-HT uptake relative to noradrenaline than
any other drug in this class of therapeutic agent.
Adverse Effects
┬Ę
Anorexia, dry mouth, nausea,
vertigo, blurred vision, tremor, drowsiness, sexual dysfunction, seizures;
suicidal ideation, mania, and paranoia; extrapyramidal effects; cardiac
arrhythmias; hyponatraemia and SIADH; and serum sickness or flu-like symptoms.
┬Ę
Serotonin
syndrome: The serotonin syndrome is a disorder that can be caused by
use of drugs or combinations of drugs which increase serotonin availability. It
most often occurs when two or more drugs which increase serotonin avail-
ability by different mechanisms are used simultaneously. Similarly, the more
severe cases tend to result from drug interactions, especially when a monoamine
oxidase inhibitor is involved. It may develop after therapeutic use or
overdose. The SSRIs may cause the development of this syndrome when used alone,
or (more commonly) when administered along with other serotonergic agents
especially monoamine oxidase inhibitors (MAOIs).
o Main
features include agitation, restlessness, confusion, disorientation,
hallucinations, drowsiness or insomnia, tachypnoea, flushing, abdominal pain,
ataxia, tremor, hypomania, myoclonus, muscle rigidity, opisthotonus, trismus,
hyperactivity, convulsions, sweating, saliva-tion, tachycardia, mydriasis,
nystagmus, teeth chat-tering, hyper- or hypotension, hyperpyrexia, coma and
diarrhoea.
o SternbachŌĆÖs diagnostic criteria for
serotonin syndrome include at least three of the following features: mental
status changes (confusion, hypomania), agitation, myoclonus, hyperreflexia,
sweating, shivering, tremor, diarrhoea, incoordination and fever.
o Hunter serotonin toxicity criteria: Hunter serotonin toxicity criteria was developed by using the Hunter Area Toxicology Service (HATS) dataset of overdoses with any serotonergic drug. Following the use/overdose of a serotonergic agent, a diagnosis of serotonin toxicity can be made if the patient meets any of the following 5 criteria:
ŌĆōŌĆō If the patient has spontaneous clonus.
ŌĆōŌĆō
If the patient has inducible clonus, and agitation or diaphoresis.
ŌĆōŌĆō
If the patient has ocular clonus, and agitation or diaphoresis.
ŌĆōŌĆō If the patient has tremor and hyperreflexia.
ŌĆōŌĆō
If the patient is hypertonic, and has a temperature greater than 38┬░C and
ocular clonus or inducible clonus.![]()
┬Ę
Any ŌĆśyesŌĆÖ decision on any of the decision rules suggests
definite or significant serotonin toxicity of sufficient clinical significance
to require consideration of treat-ment with specific 5 -HT2A
antagonists. It was found that the presence of a temperature equal or greater
than 38.5┬░C and/or marked hypertonia or rigidity (particu-larly truncal)
indicated severe serotonin toxicity with a high risk of progression to
respiratory compromise. These new criteria are simpler, more sensitive (84% vs
75%) and more specific (97% vs 96%) than SternbachŌĆÖs criteria.
┬Ę
The syndrome usually occurs in the first 2 hours of the
first dose of the drug and usually resolves within 6 to 24 hours of stoppage of
the medication. Some cases resolve even without discontinuation of the drug.
Sometimes however, complications ensue including metabolic acidosis, lactic
acidosis, rhabdomyolysis, myoglobi - nuria, renal and hepatic dysfunction, DIC,
or ARDS.
┬Ę
Hyperthermia is characteristic of serotonin syndrome. In
severe cases core temperature may exceed 42┬░C. Apart from the SSRIs, there are
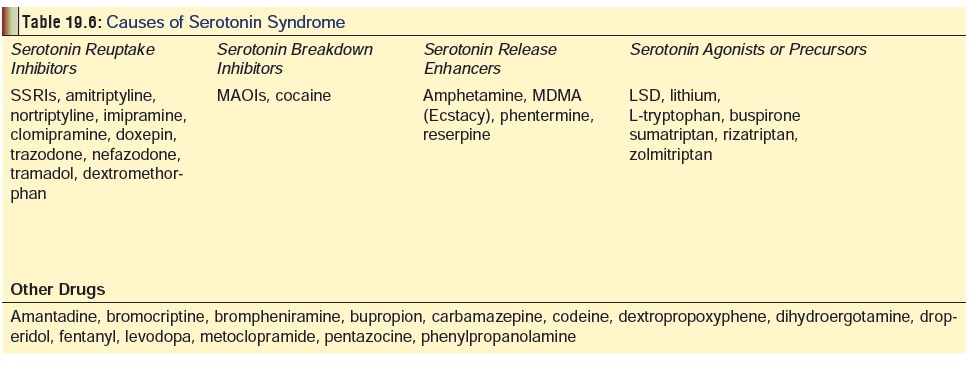
several other drugs which can cause the serotonin syndrome (Table 19.6). Serum electrolytes,
glucose, renal function tests, CK and an ECG are recommended in all patients
with suspected serotonin syndrome. Obtain liver function tests, PT/PTT or INR,
platelets, and arterial blood gases in patients with severe hyperthermia,
hypotension or other severe effects.
┬Ę
It is important to note that the serotonin syndrome has many
similarities with neuroleptic malignant syndrome (NMS). However, NMS tends to
have a slower onset and more prolonged duration of ![]() symptoms. Also, it is more
frequently associated with fever and muscle rigidity than serotonin syndrome.
On the other hand, serotonin syndrome is more likely to have myoclonus and
hyperreflexia.
symptoms. Also, it is more
frequently associated with fever and muscle rigidity than serotonin syndrome.
On the other hand, serotonin syndrome is more likely to have myoclonus and
hyperreflexia.
Treatment
of serotonin syndrome:
ŌĆōŌĆō
Benzodiazepines for agitation. ŌĆōŌĆō Rapid external cooling.
ŌĆōŌĆō
Benzodiazepines or barbiturates for convulsions. Neuromuscular blockade (with
non-depolarising paralytics) in severe cases.
ŌĆōŌĆō
Nitroprusside for severe hypertension; noradrena-line, adrenaline, or
phentolamine (NOT dopamine) for severe hypotension.
ŌĆōŌĆō
Benefit may be obtained in some cases with cypro-heptadine (4 mg/hr),
methysergide (2 mg twice daily), or propranolol. Chlorpromazine has also been
used to treat cases of serotonin syndrome.
Drug Interactions
Ō¢ĀŌ¢Ā DiazepamŌĆöConcurrent
administration of diazepam, withfluoxetine, may result in increased serum
diazepam levels due to inhibition of diazepam metabolism by fluoxetine.
Ō¢ĀŌ¢Ā MAO
inhibitorsŌĆöThe combined use of fluoxetine (andother SSRIs) with MAO
inhibitors may induce serotonin syndrome.
Ō¢ĀŌ¢Ā TricyclicsŌĆöPlasma
levels of tricyclics may be greatlyincreased when coadministered with
fluoxetine and other SSRIs. Sertraline is a weak inhibitor of several hepatic
enzymes. Inhibition appears to be dose-dependant, with higher doses, such as in
overdoses, resulting in possible clinical relevance of drug interactions,
particularly with tricyclic antidepressants (resulting in increased serum
levels of TCA and toxicity).
Clinical (Toxic) Features
┬Ę Acute SSRI overdose results in
abdominal pain, nausea, vomiting, diarrhoea, vertigo, lethargy, insomnia,
diplopia, CNS depression, tremors, and rarely convulsions.
┬Ę There is also likelihood of ECG
abnormalities (junctional rhythm, bigeminy and ventricular tachycardia, and QTc
prolongation associated with ventricular tachycardia). Left bundle branch block
has been reported with citalopram. Hypotension has also been reported.
┬Ę
Abrupt withdrawal of an SSRI after prolonged therapeutic use
may cause vertigo, nausea, vomiting, fatigue, and myalgia. A discontinuation
syndrome of dizziness, light-headedness, insomnia, fatigue, anxiety, agitation,
nausea, headache, and sensory disturbances has been described after abrupt
discontinuation of therapy with fluoxetine. A constel-lation of symptoms have
been reported following discontinu-ation of sertraline therapy. Symptoms have
included: fatigue, nausea, abdominal cramps, diarrhoea, shortness of breath,
memory impairment, dizziness, insomnia, chills, headache, eye discomfort,
tinnitus, ataxia, abnormal sensations (ŌĆ£elec-tric shocksŌĆØ, skin tingling
sensations, and involuntary move-ments). Symptoms typically resolve
spontaneously, generally within 3 weeks, or with reinstatement of sertraline
therapy.
┬Ę Paroxetine exposure in utero, with
maternal doses ranging from 20 to 120 mg/day, has resulted in a neonatal
syndrome with effects including jitteriness, vomiting, irritability,
hypoglycaemia, and necrotising enterocolitis. Withdrawal is also common in
adults; the FDA (USA) has published a new product warning concerning severe
paroxetine withdrawal effects, which could lead to drug dependency.
Treatment
┬Ę Treatment involves supportive measures.
Syrup of ipecac is contraindicated, while stomach wash is usually not
neces-sary.
┬Ę Serum levels are not clinically
useful in managing overdose.
┬Ę Monitor for evidence of serotonin
syndrome.
┬Ę Admit those with significant
clinical effects including seizures or persistent lethargy or arrhythmias.
┬Ę Sodium bicarbonate may be useful in
treating QRS prolon-gation or arrhythmias. A reasonable starting dose is 1 to 2
mEq/kg intravenous bolus, repeated as necessary. Monitor arterial blood gases
to maintain a pH of 7.45 to 7.55.
┬Ę Because of the large volume of
distribution and high degree of protein binding of SSRIs, haemodialysis, forced
diuresis, haemoperfusion and exchange transfusion would not be expected to be
useful in overdose.
Related Topics