Chapter: physics 11th 12th standard school college definition answer assignment examination viva question
Secondary Cells - Lead - Acid accumulator - Applications of secondary cells
Secondary Cells - Lead - Acid accumulator - Applications of secondary cells
Secondary Cells:
The advantage of secondary cells is that they are rechargeable. The chemical reactions that take place in secondary cells are reversible. The active materials that are used up when the cell delivers current can be reproduced by passing current through the cell in opposite direction. The chemical process of obtaining current from a secondary cell is called discharge. The process of reproducing active materials is called charging. The most common secondary cells are lead acid accumulator and alkali accumulator.
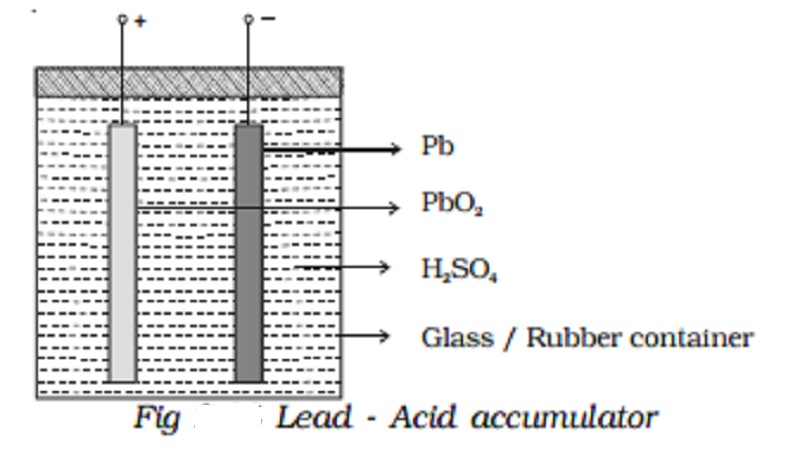
Lead - Acid accumulator :
The lead acid accumulator consists of a container made up of hard rubber or glass or celluloid. The container contains dilute sulphuric acid which acts as the electrolyte. Spongy lead (Pb) acts as the negative electrode and lead oxide (PbO2) acts as the positive electrode. The electrodes are separated by suitable insulating materials and assembled in a way to give low internal resistance.
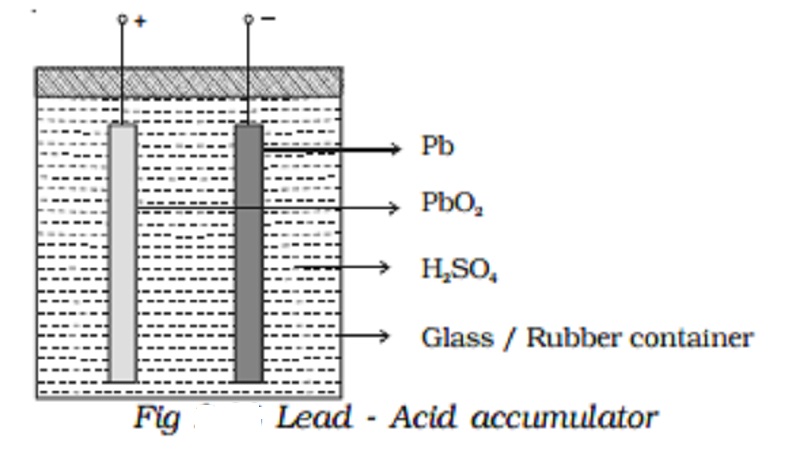
When the cell is connected in a circuit, due to the oxidation reaction that takes place at the negative electrode, spongy lead reacting with dilute sulphuric acid produces lead sulphate and two electrons. The electrons flow in the external circuit from negative electrode to positive electrode where the reduction action takes place. At the positive electrode, lead oxide on reaction with sulphuric acid produces lead sulphate and the two electrons are neutralized in this process. This makes the conventional current to flow from positive electrode to negative electrode in the external circuit.
The emf of a freshly charged cell is 2.2 Volt and the specific gravity of the electrolyte is 1.28. The cell has low internal resistance and hence can deliver high current. As the cell is discharged by drawing current from it, the emf falls to about 2 volts. In the process of charging, the chemical reactions are reversed.
Applications of secondary cells :
The secondary cells are rechargeable. They have very low internal resistance. Hence they can deliver a high current if required. They can be recharged a very large number of times without any deterioration in properties. These cells are huge in size. They are used in all automobiles like cars, two wheelers, trucks etc. The state of charging these cells is, simply monitoring the specific gravity of the electrolyte. It should lie between 1.28 to 1.12 during charging and discharging respectively.
Related Topics