Chapter: physics 11th 12th standard school college definition answer assignment examination viva question
Primary Cell - Daniel cell, Leclanche cell
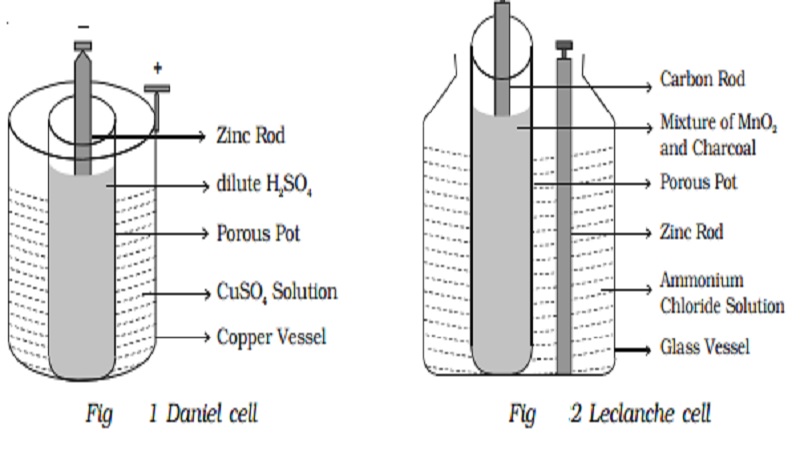
2. Primary Cell :
The cells from which the electric energy is derived by irreversible chemical actions are called primary cells. The primary cell is capable of giving an emf, when its constituents, two electrodes and a suitable electrolyte, are assembled together. The three main primary cells, namely Daniel Cell and Leclanche cell are discussed here. These cells cannot be recharged electrically.
3. Daniel cell :
Daniel cell is a primary cell which cannot supply steady current for a long time. It consists of a copper vessel containing a strong solution of copper sulphate. A zinc rod is dipped in dilute sulphuric acid contained in a porous pot. The porous pot is placed inside the copper sulphate solution.
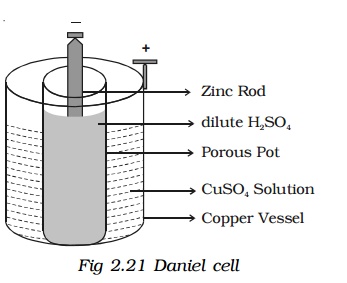
The zinc rod reacting with dilute sulphuric acid produces Zn++ ions and 2 electrons.
Zn++ ions pass through the pores of the porous pot and reacts with copper sulphate solution, producing Cu++ ions. The Cu++ ions deposit on the copper vessel. When Daniel cell is connected in a circuit, the two electrons on the zinc rod pass through the external circuit and reach the copper vessel thus neutralizing the copper ions. This constitutes an electric current from copper to zinc. Daniel cell produces an emf of 1.08 volt.
4. Leclanche cell :
A Leclanche cell consists of a carbon electrode packed in a porous pot containing manganese dioxide and charcoal powder (Fig 2.22). The porous pot is immersed in a saturated solution of ammonium chloride (electrolyte) contained in an outer glass vessel. A zinc rod is immersed in electrolytic solution.
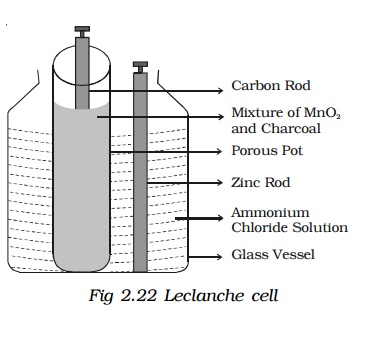
At the zinc rod, due to oxidation reaction Zn atom is converted into Zn++ ions and 2 electrons. Zn++ ions reacting with ammonium chloride produces zinc chloride and ammonia gas.
i.e Zn++ + 2 NH4Cl - 2NH3 + ZnCl2 + 2 H+ + 2e-
The ammonia gas escapes. The hydrogen ions diffuse through the pores of the porous pot and react with manganese dioxide. In this process the positive charge of hydrogen ion is transferred to carbon rod. When zinc rod and carbon rod are connected externally, the two electrons from the zinc rod move towards carbon and neutralizes the positive charge. Thus current flows from carbon to zinc.
Leclanche cell is useful for supplying intermittent current. The emf of the cell is about 1.5 V, and it can supply a current of 0.25 A.
Related Topics