Chapter: Medical Physiology: Urine Formation by the Kidneys: II. Tubular Processing of the Glomerular Filtrate
Reabsorption and Secretion Along Different Parts of the Nephron
Reabsorption and Secretion Along Different Parts of the Nephron
In the previous sections, we discussed the basic principles by which water and solutes are transported across the tubular membrane. With these generalizations in mind, we can now discuss the different characteristics of the individual tubular segments that enable them to perform their specific excretory functions. Only the tubular transport functions that are quantitatively most important are discussed, especially as they relate to the reabsorption of sodium, chloride, and water. In subsequent, we discuss the reabsorption and secretion of other specific substances in different parts of the tubular system.
Proximal Tubular Reabsorption
Normally, about 65 per cent of the filtered load of sodium and water and a slightly lower percentage of filtered chloride are reabsorbed by the proximal tubule before the filtrate reaches the loops of Henle. These percentages can be increased or decreased in different physiologic conditions, as discussed later.
Proximal Tubules Have a High Capacity for Active and Passive Reabsorption. The high capacity of the proximal tubulefor reabsorption results from its special cellular char-acteristics, as shown in Figure 27–6. The proximal tubule epithelial cells are highly metabolic and have large numbers of mitochondria to support potent active transport processes. In addition, the proximal
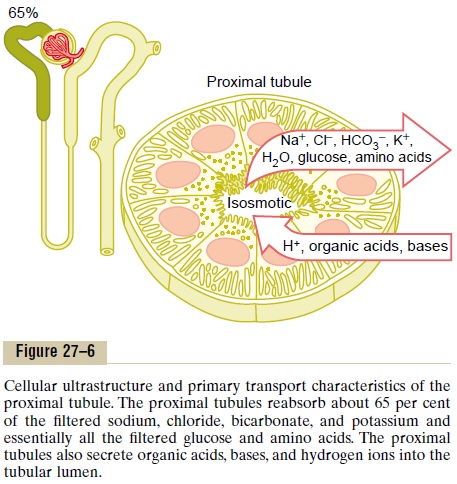
tubular cells have an extensive brush border on the luminal (apical) side of the membrane as well as an extensive labyrinth of intercellular and basal channels, all of which together provide an extensive membrane surface area on the luminal and basolateral sides of the epithelium for rapid transport of sodium ions and other substances.
The extensive membrane surface of the epithelial brush border is also loaded with protein carrier mole-cules that transport a large fraction of the sodium ions across the luminal membrane linked by way of theco-transport mechanism with multiple organic nutrientssuch as amino acids and glucose. The remainder of the sodium is transported from the tubular lumen into the cell by counter-transport mechanisms, which reabsorb sodium while secreting other substances into the tubular lumen, especially hydrogen ions. As discussed, the secretion of hydrogen ions into the tubular lumen is an important step in the removal of bicarbonate ions from the tubule (by combining H+ with the HCO3_ to form H2CO3, which then dissociates into H2O and CO2).
Although the sodium-potassium ATPase pump pro-vides the major force for reabsorption of sodium, chloride, and water throughout the proximal tubule, there are some differences in the mechanisms by which sodium and chloride are transported through the luminal side of the early and late portions of the proximal tubular membrane.
In the first half of the proximal tubule, sodium is reabsorbed by co-transport along with glucose, amino acids, and other solutes. But in the second half of the proximal tubule, little glucose and amino acids remain to be reabsorbed. Instead, sodium is now reabsorbed mainly with chloride ions.
The second half of the prox-imal tubule has a relatively high concentration of chloride (around 140 mEq/L) compared with the early proximal tubule (about 105 mEq/L) because when sodium is reabsorbed, it preferentially carries with it glucose, bicarbonate, and organic ions in the early proximal tubule, leaving behind a solution that has a higher concentration of chloride. In the second half of the proximal tubule, the higher chloride concentration favors the diffusion of this ion from the tubule lumen through the intercellular junctions into the renal inter-stitial fluid.
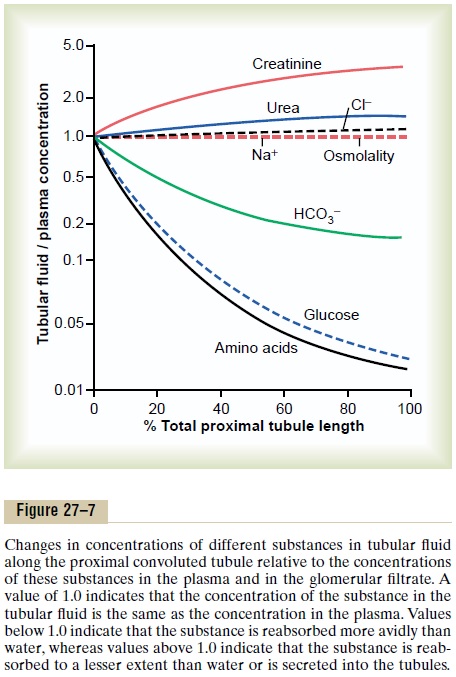
Concentrations of Solutes Along the Proximal Tubule. Figure27–7 summarizes the changes in concentrations of various solutes along the proximal tubule. Although the amount of sodium in the tubular fluid decreases markedly along the proximal tubule, the concentration of sodium (and the total osmolarity) remains relatively constant because water permeability of the proximal tubules is so great that water reabsorption keeps pace with sodium reabsorption. Certain organic solutes, such as glucose, amino acids, and bicarbonate, are much more avidly reabsorbed than water, so that their concentrations decrease markedly along the length of the proximal tubule. Other organic solutes that are less permeant and not actively reabsorbed, such as creati-nine, increase their concentration along the proximal tubule. The total solute concentration, as reflected by osmolarity, remains essentially the same all along the proximal tubule because of the extremely high per-meability of this part of the nephron to water.
Secretion of Organic Acids and Bases by the Proximal Tubule.
The proximal tubule is also an important site for secre-tion of organic acids and bases such as bile salts, oxalate, urate, and catecholamines. Many of these sub-stances are the end products of metabolism and must be rapidly removed from the body. The secretion of these substances into the proximal tubule plus filtra-tion into the proximal tubule by the glomerular capil-laries and the almost total lack of reabsorption by the tubules, all combined, contribute to rapid excretion in the urine.
In addition to the waste products of metabolism, the kidneys secrete many potentially harmful drugs or toxins directly through the tubular cells into the tubules and rapidly clear these substances from the blood. In the case of certain drugs, such as penicillin and salicylates, the rapid clearance by the kidneys creates a problem in maintaining a therapeutically effective drug concentration.
Another compound that is rapidly secreted by the proximal tubule is para-aminohippuric acid (PAH). PAH is secreted so rapidly that the average person can clear about 90 per cent of the PAH from the plasma flowing through the kidneys and excrete it in the urine. For this reason, the rate of PAH clearance can be used to estimate the renal plasma flow, as dis-cussed later.
Solute and Water Transport in the Loop of Henle
The loop of Henle consists of three functionally dis-tinct segments: the thin descending segment, the thinascending segment, and the thick ascending segment.The thin descending and thin ascending segments, as their names imply, have thin epithelial membranes with no brush borders, few mitochondria, and minimal levels of metabolic activity (Figure 27–8).
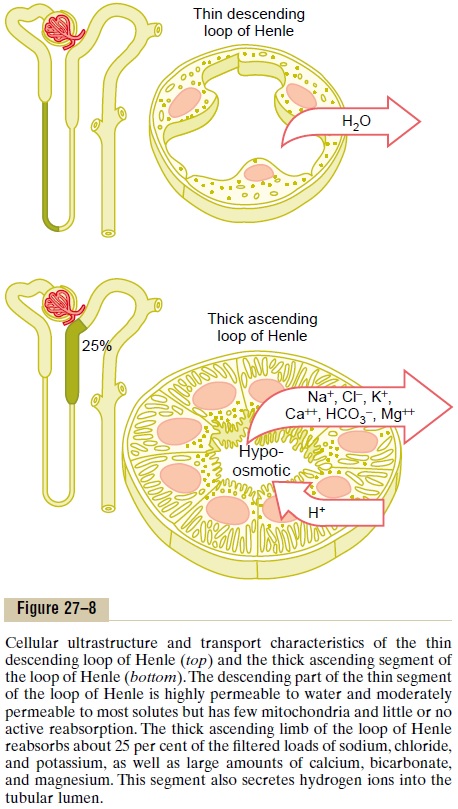
The descending part of the thin segment is highly permeable to water and moderately permeable to most solutes, including urea and sodium. The function of this nephron segment is mainly to allow simple dif-fusion of substances through its walls. About 20 per cent of the filtered water is reabsorbed in the loop of Henle, and almost all of this occurs in the thin descend-ing limb. The ascending limb, including both the thin and the thick portions, is virtually impermeable to water, a characteristic that is important for concen-trating the urine.
The thick segment of the loop of Henle, which begins about halfway up the ascending limb, has thick epithelial cells that have high metabolic activity and are capable of active reabsorption of sodium, chloride, and potassium (see Figure 27–8). About 25 per cent of the filtered loads of sodium, chloride, and potassium are reabsorbed in the loop of Henle, mostly in the thick ascending limb. Considerable amounts of other ions, such as calcium, bicarbonate, and magnesium, are also reabsorbed in the thick ascending loop of Henle.
The thin segment of the ascending limb has a much lower reabsorptive capacity than the thick segment, and the thin descending limb does not reabsorb sig-nificant amounts of any of these solutes.
An important component of solute reabsorption in the thick ascending limb is the sodium-potassium ATPase pump in the epithelial cell basolateral mem-branes. As in the proximal tubule, the reabsorption of other solutes in the thick segment of the ascending loop of Henle is closely linked to the reabsorptive capability of the sodium-potassium ATPase pump, which maintains a low intracellular sodium concentra-tion. The low intracellular sodium concentration in turn provides a favorable gradient for movement of sodium from the tubular fluid into the cell. In thethick ascending loop, movement of sodium across the luminal membrane is mediated primarily by a 1-sodium, 2-chloride, 1-potassium co-transporter (Figure 27–9). This co-transport protein carrier in the luminal membrane uses the potential energy released by downhill diffusion of sodium into the cell to drive the reabsorption of potassium into the cell against a concentration gradient.
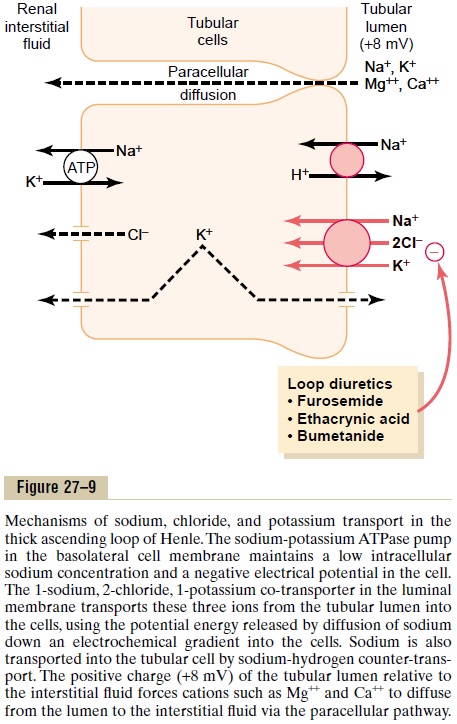
The thick ascending limb of the loop of Henle is the site of action of the powerful “loop” diureticsfurosemide, ethacrynic acid, and bumetanide, all ofwhich inhibit the action of the sodium 2-chloride, potassium co-transporter.
There is also significant paracellular reabsorption of cations, such as Mg++, Ca++, Na+, and K+, in the thick ascending limb owing to the slight positive charge of the tubular lumen relative to the interstitial fluid. Although the 1-sodium, 2-chloride, 1-potassium co-transporter moves equal amounts of cations and anions into the cell, there is a slight backleak of potas-sium ions into the lumen, creating a positive charge of about +8 millivolts in the tubular lumen. This positive charge forces cations such as Mg++ and Ca++ to diffuse from the tubular lumen through the paracellular space and into the interstitial fluid.
The thick ascending limb also has a sodium-hydrogen counter-transport mechanism in its luminal cell membrane that mediates sodium reabsorption and hydrogen secretion in this segment.
The thick segment of the ascending loop of Henle is virtually impermeable to water. Therefore, most of thewater delivered to this segment remains in the tubule, despite reabsorption of large amounts of solute. The tubular fluid in the ascending limb becomes very dilute as it flows toward the distal tubule, a feature that is important in allowing the kidneys to dilute or concen-trate the urine under different conditions.
Distal Tubule
The thick segment of the ascending limb of the loop of Henle empties into the distal tubule. The very first portion of the distal tubule forms part of the juxta-glomerular complex that provides feedback control ofGFR and blood flow in this same nephron. The next part of the distal tubule is highly convoluted and has many of the same reabsorptive characteristics of the thick segment of the ascending limb of the loop of Henle. That is, it avidly reabsorbs most of the ions, including sodium, potassium, and chloride, but is vir-tually impermeable to water and urea. For this reason, it is referred to as the diluting segmentbecause it also dilutes the tubular fluid.
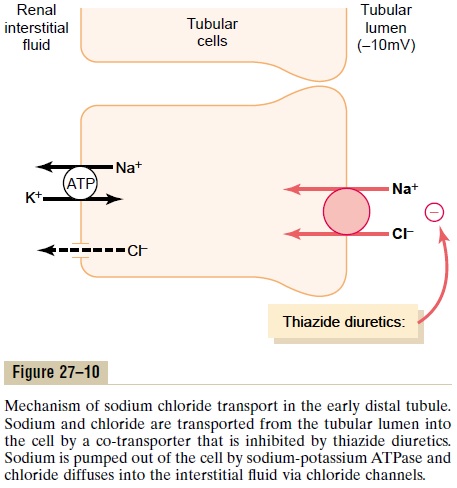
Approximately 5 percent of the filtered load of sodium chloride is reabsorbed in the early distal tubule. The sodium-chloride co-transporter moves sodium chloride from the tubular lumen into the cell, and the sodium-potassium ATPase pump transports sodium out of the cell across the basolateral mem-brane (Figure 27–10). Chloride diffuses out of the cell into the renal interstitial fluid through chloride chan-nels in the basolateral membrane. The thiazide diuret-ics, which are widely used to treat disorders such ashypertension and heart failure, inhibit the sodium-chloride co-transporter.
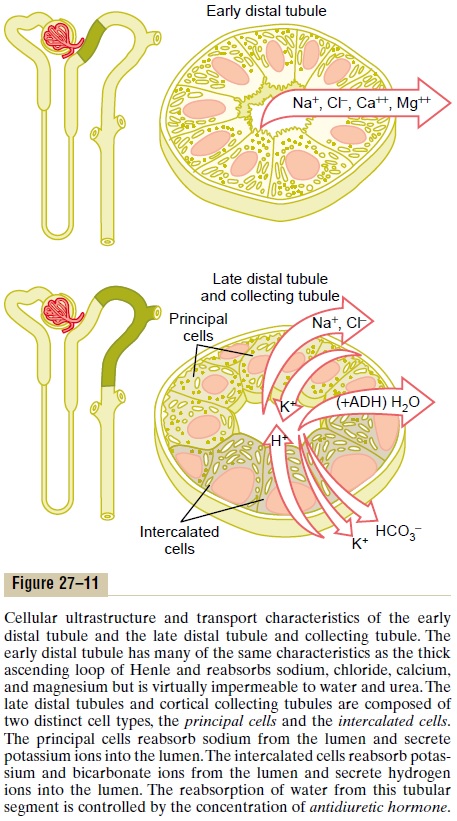
Late Distal Tubule and Cortical Collecting Tubule
The second half of the distal tubule and the subse-quent cortical collecting tubule have similar functional characteristics. Anatomically, they are composed of two distinct cell types, the principal cells and the inter-calated cells (Figure 27–11). The principal cells reab-sorb sodium and water from the lumen and secrete potassium ions into the lumen. The intercalated cells reabsorb potassium ions and secrete hydrogen ions into the tubular lumen.
Principal Cells Reabsorb Sodium and Secrete Potassium.
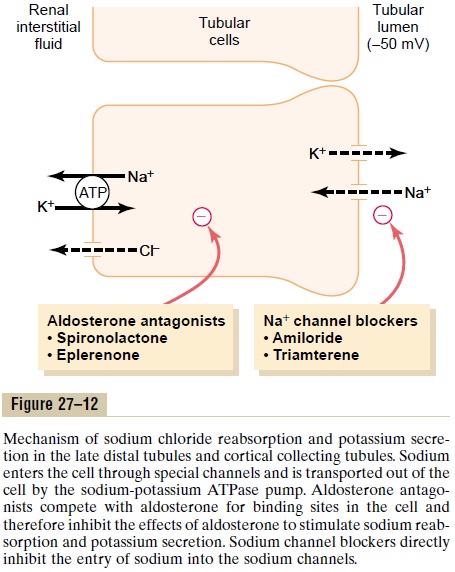
Sodium reabsorption and potassium secretion by the principal cells depend on the activity of a sodium-potassium ATPase pump in each cell’s basolateral membrane (Figure 27–12). This pump maintains a low sodium concentration inside the cell and, therefore, favors sodium diffusion into the cell through special
The secretion of potassium by these cells from the blood into the tubular lumen involves two steps: (1) Potassium enters the cell because of the sodium-potassium ATPase pump, which maintains a high intracellular potassium concentration, and then (2) once in the cell, potassium diffuses down its con-centration gradient across the luminal membrane into the tubular fluid.
The principal cells are the primary sites of action of the potassium-sparing diuretics, including spirono-lactone, eplerenone, amiloride, and triamterene. Aldosterone antagonists compete with aldosterone for receptor sites in the principal cells and therefore inhibit the stimulatory effects of aldosterone on sodium reabsorption and potassium secretion. Sodium channel blockers directly inhibit the entry of sodium into the sodium channels of the luminal membranes and therefore reduce the amount of sodium that can be transported across the basolateral membranes by the sodium-potassium ATPase pump. This, in turn, decreases transport of potassium into the cells and ultimately reduces potassium secretion into the tubular fluid. For this reason the sodium channel blockers as well as the aldosterone antagonists decrease urinary excretion of potassium and act as potassium-sparing diuretics.
Intercalated Cells Avidly Secrete Hydrogen and Reabsorb Bicar-bonate and Potassium Ions. Hydrogen ion secretion bythe intercalated cells is mediated by a hydrogen-ATPase transport mechanism. Hydrogen is generated in this cell by the action of carbonic anhydrase on water and carbon dioxide to form carbonic acid, which then dissociates into hydrogen ions and bicarbonate ions. The hydrogen ions are then secreted into the tubular lumen, and for each hydrogen ion secreted, a bicarbonate ion becomes available for reabsorption across the basolateral membrane. The intercalated cells can also reabsorb potassium ions.
The functional characteristics of the late distal tubule and cortical collecting tubule can be summarized as follows:
1. The tubular membranes of both segments are almost completely impermeable to urea, similar to the diluting segment of the early distal tubule; thus, almost all the urea that enters these segments passes on through and into the collecting duct to be excreted in the urine, although some reabsorption of urea occurs in the medullary collecting ducts.
2. Both the late distal tubule and the cortical collecting tubule segments reabsorb sodium ions, and the rate of reabsorption is controlled by hormones, especially aldosterone. At the same time, these segments secrete potassium ions from the peritubular capillary blood into the tubular lumen, a process that is also controlled by aldosterone and by other factors such as the concentration of potassium ions in the body fluids.
3. The intercalated cells of these nephron segments avidly secrete hydrogen ions by an active hydrogen-ATPase mechanism. This process is different from the secondary active secretion of hydrogen ions by the proximal tubule because it is capable of secreting hydrogen ions against a large concentration gradient, as much as 1000 to 1. This is in contrast to the relatively small gradient (4- to 10-fold) for hydrogen ions that can be achieved by secondary active secretion in the proximal tubule. Thus, the intercalated cells play a key role in acid-base regulation of the body fluids.
4. The permeability of the late distal tubule and cortical collecting duct to water is controlled by the concentration of ADH, which is also called vasopressin. With high levels of ADH, thesetubular segments are permeable to water, but in the absence of ADH, they are virtually impermeable to water. This special characteristic provides an important mechanism for controlling the degree of dilution or concentration of the urine.
Medullary Collecting Duct
Although the medullary collecting ducts reabsorb less than 10 per cent of the filtered water and sodium, they are the final site for processing the urine and, there-fore, play an extremely important role in determining the final urine output of water and solutes.
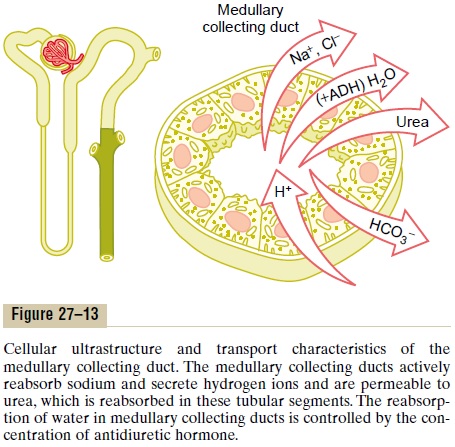
The epithelial cells of the collecting ducts are nearly cuboidal in shape with smooth surfaces and relatively few mitochondria (Figure 27–13). Special characteris-tics of this tubular segment are as follows:
1. The permeability of the medullary collecting duct to water is controlled by the level of ADH. With high levels of ADH, water is avidly reabsorbed into the medullary interstitium, thereby reducing the urine volume and concentrating most of the solutes in the urine.
2. Unlike the cortical collecting tubule, the medullary collecting duct is permeable to urea. Therefore, some of the tubular urea is reabsorbed into the medullary interstitium, helping to raise the osmolality in this region of the kidneys and contributing to the kidneys’ overall ability to form a concentrated urine.
3. The medullary collecting duct is capable of secreting hydrogen ions against a large concentration gradient, as also occurs in the cortical collecting tubule. Thus, the medullary collecting duct also plays a key role in regulating acid-base balance.
Summary of Concentrations of Different Solutes in the Different Tubular Segments
Whether a solute will become concentrated in the tubular fluid is determined by the relative degree of reabsorption of that solute versus the reabsorption of water. If a greater percentage of water is reabsorbed, the substance becomes more concentrated. If a greater percentage of the solute is reabsorbed, the substance becomes more diluted.
Figure 27–14 shows the degree of concentration of several substances in the different tubular segments.All the values in this figure represent the tubular fluid concentration divided by the plasma concentration of a substance. If plasma concentration of the substance is assumed to be constant, any change in the ratio of tubular fluid/plasma concentration rate reflects changes in tubular fluid concentration.
As the filtrate moves along the tubular system, the concentration rises to progressively greater than 1.0 if more water is reabsorbed than solute, or if there has been a net secretion of the solute into the tubular fluid. If the concentration ratio becomes progressively less than 1.0, this means that relatively more solute has been reabsorbed than water.
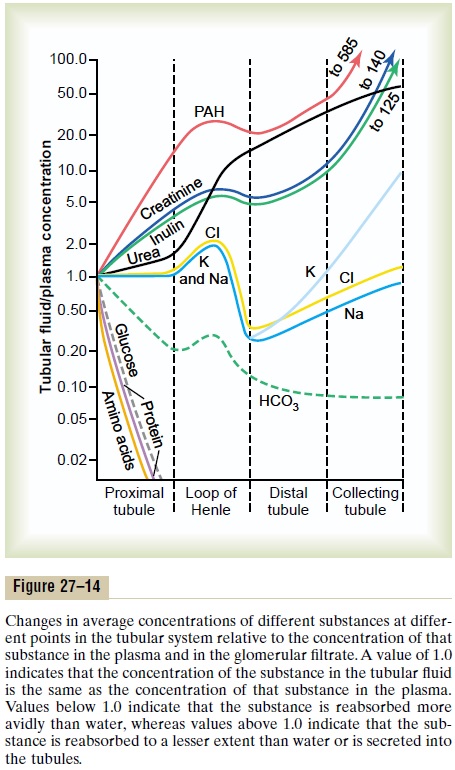
The substances represented at the top of Figure 27–14, such as creatinine, become highly concentrated in the urine. In general, these substances are not needed by the body, and the kidneys have become adapted to reabsorb them only slightly or not at all, or even to secrete them into the tubules, thereby excreting especially great quantities into the urine. Conversely, the substances represented toward the bottom of the figure, such as glucose and amino acids, are all strongly reabsorbed; these are all substances that the body needs to conserve, and almost none of them are lost in the urine.
Tubular Fluid /Plasma Inulin Concentration Ratio Can Be Used to Measure Water Reabsorption by the Renal Tubules. Inulin,a polysaccharide used to measure GFR, is not reab-sorbed or secreted by the renal tubules. Changes in inulin concentration at different points along the renal tubule, therefore, reflect changes in the amount of water present in the tubular fluid. For example, the tubular fluid/plasma concentration ratio for inulin rises to about 3.0 at the end of the proximal tubules, indicating that inulin concentration in the tubular fluid is 3 times greater than in the plasma and in the glomerular filtrate. Since inulin is not secreted or reab-sorbed from the tubules, a tubular fluid/plasma con-centration ratio of 3.0 means that only one third of the water that was filtered remains in the renal tubule and that two thirds of the filtered water has been reab-sorbed as the fluid passes through the proximal tubule. At the end of the collecting ducts, the tubular fluid/plasma inulin concentration ratio rises to about 125 (see Figure 27–14), indicating that only 1/125 of the filtered water remains in the tubule and that more than 99% has been reabsorbed.
Related Topics