Chapter: Medical Physiology: Regulation of Acid-Base Balance
Quantitative Dynamics of the Bicarbonate Buffer System
Quantitative Dynamics of the Bicarbonate Buffer System
All acids, including H2CO3, are ionized to some extent. From mass balance considerations, the concentrations of H+ and HCO3– are proportional to the concentration of H2CO3.
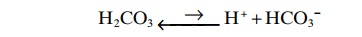
For any acid, the concentration of the acid relative to its dissociated ions is defined by the dissociationconstant K'.
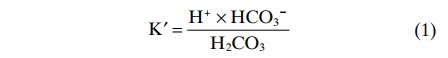
This equation indicates that in an H2CO3 solution, the amount of free H+ is equal to
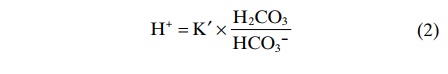
The concentration of undissociated H2CO3 cannot be measured in solution because it rapidly dissociates into CO2 and H2O or to H+and HCO3-. However, the CO2 dissolved in the blood is directly proportional to the amount of undissociated H2CO3. Therefore, equation 2 can be rewritten as
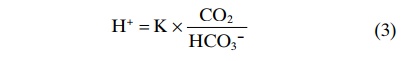
The dissociation constant (K) for equation 3 is only about 1/400 of the dissociation constant (K’) of equation 2 because the proportionality ratio between H2CO3 and CO2 is 1:400. Equation 3 is written in terms of the total amount of CO2 dissolved in solution. However, most clinical laboratories measure the blood CO2 tension (PCO2) rather than the actual amount of CO2. Fortunately, the amount of CO2 in the blood is a linear function of PCO2 times the solubility coefficient for CO2; under physiologic conditions, the solubility coefficient for CO2 is 0.03 mmol/mm Hg at body temperature.This means that 0.03 millimole of H2CO3 is present in the blood for each millimeter of mercury PCO2 measured. Therefore, equation 3 can be rewritten as
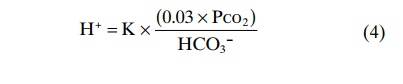
Henderson-Hasselbalch Equation. As discussed earlier, it is customary to express H+ concentration in pH units rather than in actual concentrations. Recall that pH is defined as pH = –log H+
The dissociation constant can be expressed in a similar manner.
pK = –log K
Therefore, we can express the H+ concentration in equation 4 in pH units by taking the negative logarithm of that equation, which yields
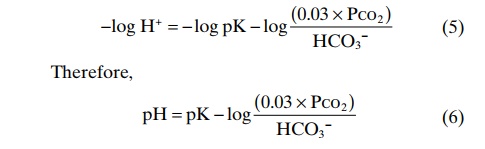
Rather than work with a negative logarithm, we can change the sign of the logarithm and invert the numerator and denominator in the last term, using the law of logarithms to yield
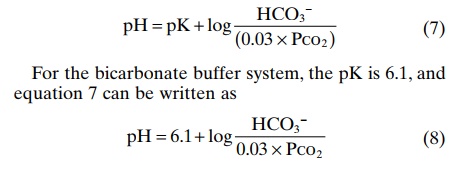
Equation 8 is the Henderson-Hasselbalch equation, and with it, one can calculate the pH of a solution if the molar concentration of HCO3– and the PCO2 are known. From the Henderson-Hasselbalch equation, it is apparent that an increase in HCO3– concentration causes the pH to rise, shifting the acid-base balance toward alkalosis. An increase in PCO2 causes the pH to decrease, shifting the acid-base balance toward acidosis.
The Henderson-Hasselbalch equation, in addition to defining the determinants of normal pH regulation and defining the determinants of normal pH regulation and acid-base balance in the extracellular fluid, provides insight into the physiologic control of acid and base composition of the extracellular fluid. As discussed later, the bicarbonate concentration is regulated mainly by the kidneys, whereas the PCO2 in extracellular fluid is controlled by the rate of respiration. By increasing the rate of respiration, the lungs remove CO2from the plasma, and by decreasing respiration, the lungs elevate PCO2. Normal physiologic acid-base homeostasis results from the coordinated efforts of both of these organs, the lungs and the kidneys, and acid-base disorders occur when one or both of these control mechanisms are impaired, thus altering either the bicarbonate concentration or the PCO2 of extracellular fluid.
When disturbances of acid-base balance result from a primary change in extracellular fluid bicarbonate concentration, they are referred to as metabolic acid-base disorders. Therefore, acidosis caused by a primary decrease in bicarbonate concentration is termed metabolic acidosis, whereas alkalosis caused by a primary increase in bicarbonate concentration is called metabolic alkalosis. Acidosis caused by an increase in PCO2 is called respiratory acidosis, whereas alkalosis caused by a decrease in PCO2 is termed respiratory alkalosis.
Bicarbonate Buffer System Titration Curve. Figure 30–1 shows the changes in pH of the extracellular fluid when the ratio of HCO3 – to CO2 in extracellular fluid is altered. When the concentrations of these two components are equal, the right-hand portion of equation 8 becomes the log of 1, which is equal to 0. Therefore, when the two components of the buffer system are equal, the pH of the solution is the same as the pK (6.1) of the bicarbonate buffer system.When base is added to the system, part of the dissolved CO2is converted into HCO3 causing an increase in the ratio of HCO3 – to CO2 and increasing the pH, as is evident from the HendersonHasselbalch equation. When acid is added, it is buffered by HCO3–, which is then converted into dissolved CO2, decreasing the ratio of HCO3– to CO2 and decreasing the pH of the extracellular fluid.
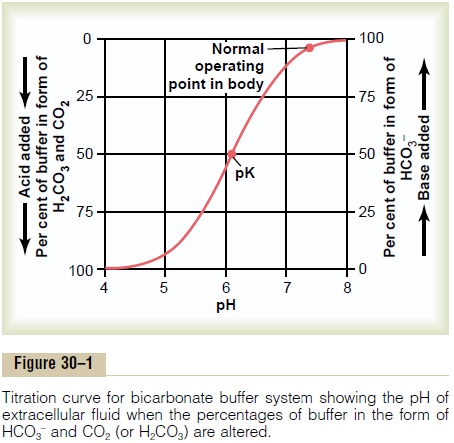
“Buffer Power” Is Determined by the Amount and Relative Con- centrations of the Buffer Components. From the titrationcurve in Figure 30–1, several points are apparent. First, the pH of the system is the same as the pK when each of the components (HCO3– and CO2) constitutes 50 per cent of the total concentration of the buffer system. Second, the buffer system is most effective in the central part of the curve, where the pH is near the pK of the system. This means that the change in pH for any given amount of acid or base added to the system is least when the pH is near the pK of the system. The buffer system is still reasonably effective for 1.0 pH unit on either side of the pK, which for the bicarbonate buffer system extends from a pH of about 5.1 to 7.1 units. Beyond these limits, the buffering power rapidly diminishes. And when all the CO2 has been converted into HCO3– or when all the HCO3– has been converted into CO2, the system has no more buffering power.
The absolute concentration of the buffers is also an important factor in determining the buffer power of a system. With low concentrations of the buffers, only a small amount of acid or base added to the solution changes the pH considerably.
Bicarbonate Buffer System Is the Most Important Extracellular Buffer. From the titration curve shown in Figure 30–1,one would not expect the bicarbonate buffer system to be powerful, for two reasons: First, the pH of the extra-cellular fluid is about 7.4, whereas the pK of the bicar-bonate buffer system is 6.1. This means that there is about 20 times as much of the bicarbonate buffer system in the form of HCO3– as in the form of dis-solved CO2. For this reason, this system operates on the portion of the buffering curve where the slope is low and the buffering power is poor. Second, the con-centrations of the two elements of the bicarbonate system, CO2 and HCO3–, are not great.
Despite these characteristics, the bicarbonate buffer system is the most powerful extracellular buffer in the body. This apparent paradox is due mainly to the fact that the two elements of the buffer system, HCO3– and CO2, are regulated, respectively, by the kidneys and the lungs, as discussed later. As a result of this regulation, the pH of the extracellular fluid can be precisely con-trolled by the relative rate of removal and addition of HCO3– by the kidneys and the rate of removal of CO2 by the lungs.
Related Topics