Chapter: Medical Physiology: Regulation of Acid-Base Balance
Buffering of Hydrogen Ions in the Body Fluids
Buffering of Hydrogen Ions in the Body Fluids
A buffer is any substance that can reversibly bind H+. The general form of the buffering reaction is
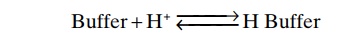
In this example, a free H+ combines with the buffer to form a weak acid (H buffer) that can either remain as an unassociated molecule or dissociate back to buffer and H+. When the H+ concentration increases, the reaction is forced to the right, and more H+ binds to the buffer, as long as buffer is available. Conversely, when the H+ concentration decreases, the reaction shifts toward the left, and H+is released from the buffer. In this way, changes in H+ concentration are minimized.
The importance of the body fluid buffers can be quickly realized if one considers the low concentration of H+ in the body fluids and the relatively large amounts of acids produced by the body each day. For example, about 80 milliequivalents of hydrogen is either ingested or produced each day by metabolism, whereas the H+ concentration of the body fluids nor-mally is only about 0.00004 mEq/L. Without buffering, the daily production and ingestion of acids would cause huge changes in body fluid H+ concentration.
The action of acid-base buffers can perhaps best be explained by considering the buffer system that is quantitatively the most important in the extracellular fluid—the bicarbonate buffer system.
Related Topics