Chapter: Medical Physiology: Regulation of Acid-Base Balance
Acidosis Decreases the Ratio of HCO3-/H+ in Renal Tubular Fluid
Renal Correction of Acidosis- Increased Excretion of Hydrogen Ions and Addition of Bicarbonate Ions to the Extracellular Fluid
Now that we have described the mechanisms by which the kidneys secrete H+ and reabsorb HCO3–, we can explain how the kidneys readjust the pH of the extra-cellular fluid when it becomes abnormal.
Referring to equation 8, the Henderson-Hasselbalch equation, we can see that acidosis occurs when the ratio of HCO3– to CO2 in the extracellular fluid decreases, thereby decreasing pH. If this ratio decreases because of a fall in HCO3–, the acidosis is referred to asmetabolic acidosis. If the pH falls because of an increase in PCO2, the acidosis is referred to as respiratory acidosis.
Acidosis Decreases the Ratio of HCO3-/H+ in Renal Tubular Fluid
Both respiratory and metabolic acidosis cause a decrease in the ratio of HCO3– to H+ in the renal tubular fluid. As a result, there is an excess of H+ in the renal tubules, causing complete reabsorption of HCO3– and still leaving additional H+ available to combine with the urinary buffers NH4+ and HPO4=. Thus, in aci-dosis, the kidneys reabsorb all the filtered HCO3– and contribute new HCO3– through the formation of NH4+ and titratable acid.
In metabolic acidosis, an excess of H + over HCO3– occurs in the tubular fluid primarily because of decreased filtration of HCO3-.This decreasedfiltrationof HCO3– is caused mainly by a decrease in the extra-cellular fluid concentration of HCO3–.
In respiratory acidosis, the excess H+ in the tubular fluid is due mainly to the rise in extracellular fluid PCO2, which stimulates H+secretion.
As discussed previously, with chronic acidosis, regardless of whether it is respiratory or metabolic, there is an increase in the production of NH4+, which further contributes to the excretion of H+ and the addi-tion of new HCO3– to the extracellular fluid. With severe chronic acidosis, as much as 500 mEq/day of H+ can be excreted in the urine, mainly in the form of NH4+; this, in turn, contributes up to 500 mEq/day of new HCO3– that is added to the blood.
Thus, with chronic acidosis, the increased secretion of H+ by the tubules helps eliminate excess H+ from the body and increases the quantity of HCO3– in the extracellular fluid. This increases the bicarbonate part of the bicarbonate buffer system, which, in accordance with the Henderson-Hasselbalch equation, helps raise the extracellular pH and corrects the acidosis. If the acidosis is metabolically mediated, additional com-pensation by the lungs causes a reduction in PCO2, also helping to correct the acidosis.
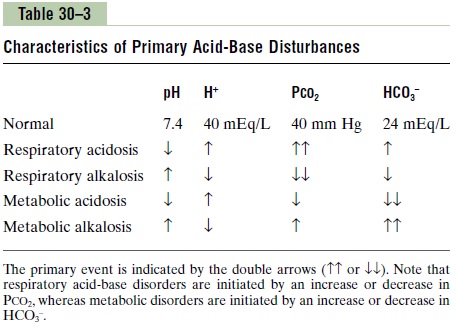
Table 30–3 summarizes the characteristics associated with respiratory and metabolic acidosis as well as respiratory and metabolic alkalosis, which are dis-cussed in the next section. Note that in respiratoryacidosis, there is a reduction in pH, an increase inextracellular fluid H+ concentration, and an increase in PCO2, which is the initial cause of the acidosis. Thecompensatory response is an increase in plasma HCO3-, caused by the addition of new bicarbonate to the extracellular fluid by the kidneys. The rise in HCO3–helps offset the increase in PCO2, thereby returning the plasma pH toward normal.
In metabolic acidosis, there is also a decrease in pH and a rise in extracellular fluid H+ concentration. However, in this case, the primary abnormality is a decrease in plasma HCO3–. The primary compensa-tions include increased ventilation rate, which reduces PCO2 , and renal compensation, which, by adding new bicarbonate to the extracellular fluid, helps minimize the initial fall in extracellular HCO3- concentration.
Related Topics