Chapter: Medical Physiology: Regulation of Acid-Base Balance
Combination of Excess Hydrogen Ions with Phosphate and Ammonia Buffers in the Tubule-A Mechanism for Generating “New” Bicarbonate Ions
Combination of Excess Hydrogen Ions with Phosphate and Ammonia Buffers in the Tubule-A Mechanism for Generating “New” Bicarbonate Ions
When H+ is secreted in excess of the bicarbonate fil-tered into the tubular fluid, only a small part of the excess H+ can be excreted in the ionic form (H+) in the urine. The reason for this is that the minimal urine pH is about 4.5, corresponding to an H+ concentration of 10–4.5 mEq/L, or 0.03 mEq/L. Thus, for each liter of urine formed, a maximum of only about 0.03 mil-liequivalent of free H+ can be excreted. To excrete the 80 milliequivalents of nonvolatile acid formed by metabolism each day, about 2667 liters of urine would have to be excreted if the H+ remained free in solution.
The excretion of large amounts of H+ (on occasion as much as 500 mEq/day) in the urine is accomplished primarily by combining the H+ with buffers in the tubular fluid. The most important buffers are phos-phate buffer and ammonia buffer. There are other weak buffer systems, such as urate and citrate, that are much less important.
When H+ is titrated in the tubular fluid with HCO3–, this results in the reabsorption of one HCO3– for each H+ secreted, as discussed earlier. But when there are excess H+ in the urine, they combine with buffers other than HCO3–, and this results in the generation of new HCO3– that can also enter the blood. Thus, when there is excess H+ in the extracellular fluid, the kidneys not only reabsorb all the filtered HCO3– but also generate new HCO3–, thereby helping to replenish the HCO3– lost from the extracellular fluid in acidosis. In the next two sections, we discuss the mechanisms by which phosphate and ammonia buffers contribute to the gen-eration of new HCO3–.
Phosphate Buffer System Carries Excess Hydrogen Ions into the Urine and Generates New Bicarbonate
The phosphate buffer system is composed of HPO4= and H2PO4–. Both become concentrated in the tubular fluid because of their relatively poor reabsorption and because of the reabsorption of water from the tubular fluid. Therefore, although phosphate is not an impor-tant extracellular fluid buffer, it is much more effective as a buffer in the tubular fluid.
Another factor that makes phosphate important as a tubular buffer is the fact that the pK of this system is about 6.8. Under normal conditions, the urine is slightly acidic, and the urine pH is near the pK of the phosphate buffer system. Therefore, in the tubules, the phosphate buffer system normally functions near its most effective range of pH.
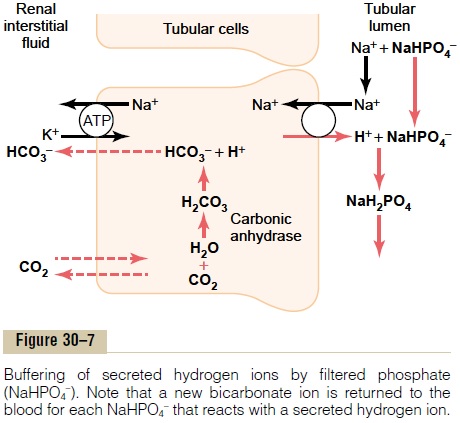
Figure 30–7 shows the sequence of events by which H+ is excreted in combination with phosphate buffer and the mechanism by which new bicarbonate is added to the blood. The process of H+ secretion into the tubules is the same as described earlier. As long as there is excess HCO3– in the tubular fluid, most of the secreted H+ combines with HCO3–. However, once all the HCO3– has been reabsorbed and is no longer avail-able to combine with H+, any excess H+ can combine with HPO4= and other tubular buffers. After the H+ combines with HPO4= to form H2PO4–, it can be excreted as a sodium salt (NaH2PO4), carrying with it the excess hydrogen.
There is one important difference in this sequence of H+ excretion from that discussed previously. In this case, the HCO3– that is generated in the tubular cell and enters the peritubular blood represents a net gain of HCO3– by the blood, rather than merely a replace-ment of filtered HCO3–. Therefore, whenever an H+secreted into the tubular lumen combines with a buffer other than HCO3-, the net effect is addition of a new HCO3- to the blood. This demonstrates one of themechanisms by which the kidneys are able to replen-ish the extracellular fluid stores of HCO3–.
Under normal conditions, much of the filtered phos-phate is reabsorbed, and only about 30 to 40 mEq/day is available for buffering H+. Therefore, much of the buffering of excess H+ in the tubular fluid in acidosis occurs through the ammonia buffer system.
Excretion of Excess Hydrogen Ions and Generation of New Bicarbonate by the Ammonia Buffer System
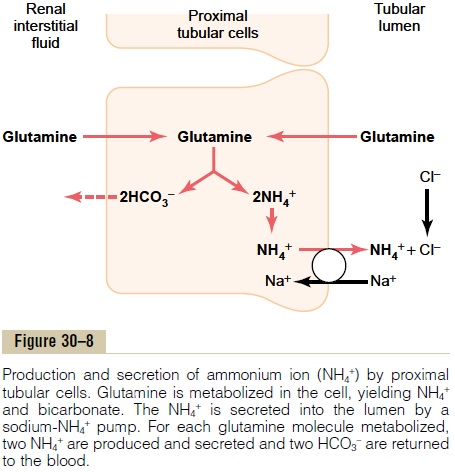
A second buffer system in the tubular fluid that is even more important quantitatively than the phosphate buffer system is composed of ammonia (NH3) and the ammonium ion (NH4+). Ammonium ion is synthesized from glutamine, which comes mainly from the metab-olism of amino acids in the liver. The glutamine deliv-ered to the kidneys is transported into the epithelial cells of the proximal tubules, thick ascending limb of the loop of Henle, and distal tubules (Figure 30–8). Once inside the cell, each molecule of glutamine is metabolized in a series of reactions to ultimately form two NH4+ and two HCO3–. The NH4+ is secreted into the tubular lumen by a counter-transport mechanism in exchange for sodium, which is reabsorbed. The HCO3– is transported across the basolateral mem-brane, along with the reabsorbed Na+, into the inter-stitial fluid and is taken up by the peritubular capillaries. Thus, for each molecule of glutamine metabolized in the proximal tubules, two NH4+ are secreted into the urine and two HCO3– are reabsorbed into the blood. The HCO3–generated by this processconstitutes new bicarbonate.
In the collecting tubules, the addition of NH4+ to the tubular fluids occurs through a different mechanism (Figure 30–9). Here, H+ is secreted by the tubular
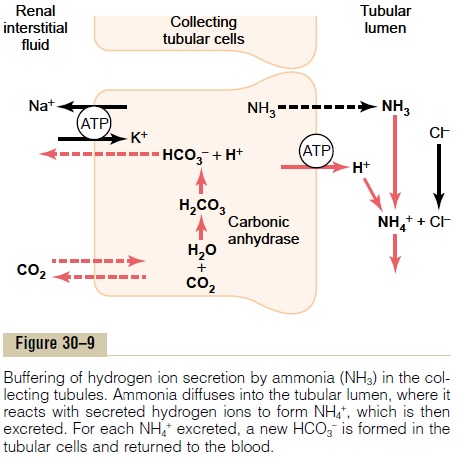
membrane into the lumen, where it combines with NH3 to form NH4+, which is then excreted. The col-lecting ducts are permeable to NH3, which can easily diffuse into the tubular lumen. However, the luminal membrane of this part of the tubules is much less per-meable to NH4+; therefore, once the H+ has reacted with NH3 to form NH4+, the NH4+ is trapped in the tubular lumen and eliminated in the urine. For eachNH4+ excreted, a new HCO3- is generated and added to the blood.
Chronic Acidosis Increases NH4+ Excretion. One of the mostimportant features of the renal ammonium-ammonia buffer system is that it is subject to physiologic control.
An increase in extracellular fluid H+ concentration stimulates renal glutamine metabolism and, therefore, increases the formation of NH4+ and new HCO3– to be used in H+ buffering; a decrease in H+concentration has the opposite effect.
Under normal conditions, the amount of H+ elimi-nated by the ammonia buffer system accounts for about 50 per cent of the acid excreted and 50 per cent of the new HCO3– generated by the kidneys. However, with chronic acidosis, the rate of NH4+ excretion can increase to as much as 500 mEq/day. Therefore, withchronic acidosis, the dominant mechanism by which acid is eliminated is excretion of NH4+. This also pro-vides the most important mechanism for generating new bicarbonate during chronic acidosis.
Related Topics