Chapter: Medical Physiology: Regulation of Acid-Base Balance
Clinical Measurements and Analysis of Acid-Base Disorders
Clinical Measurements and Analysis of Acid-Base Disorders
Appropriate therapy of acid-base disorders requires proper diagnosis. The simple acid-base disorders described previously can be diagnosed by analyzing three measurements from an arterial blood sample: pH, plasma bicarbonate concentration, and PCO2.
The diagnosis of simple acid-base disorders involves several steps, as shown in Figure 30–10. By exam-ining the pH, one can determine whether the disorder is acidosis or alkalosis. A pH less than 7.4 indicates acidosis, whereas a pH greater than 7.4 indicates alkalosis.
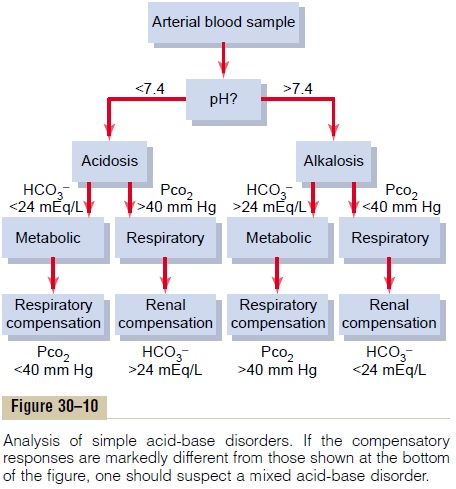
The second step is to examine the plasma PCO2 and HCO3– concentration. The normal value for PCO2 is about 40 mm Hg, and for HCO3–, it is 24 mEq/L. If the disorder has been characterized as acidosis and the plasma PCO2 is increased, there must be a respiratory component to the acidosis. After renal compensation, the plasma HCO3– concentration in respiratory acidosis would tend to increase above normal. Therefore, theexpected values for a simple respiratory acidosis would be reduced plasma pH, increased PCO2 , and increased plasma HCO3– concentration after partial renal compensation.
For metabolic acidosis, there would also be a decrease in plasma pH. However, with metabolic acidosis, the primary abnormality is a decrease in plasma HCO3– concentration. Therefore, if a low pH is associated with a low HCO3– concentration, there must be a metabolic component to the acidosis. In simple metabolic acidosis, the PCO2 is reduced because of partial respiratory com-pensation, in contrast to respiratory acidosis, in which PCO2 is increased. Therefore, in simple metabolic acido-sis, one would expect to find a low pH, a low plasma HCO3- concentration, and a reduction in PCO2 after partial respiratory compensation.
The procedures for categorizing the types of alkalo-sis involve the same basic steps. First, alkalosis implies that there is an increase in plasma pH. If the increase in pH is associated with decreased PCO2, there must be a respiratory component to the alkalosis. If the rise in pH is associated with increased HCO3–, there must be a metabolic component to the alkalosis. Therefore, insimple respiratory alkalosis, one would expect to find increased pH, decreased PCO2 , and decreased HCO3-concentration in the plasma. In simple metabolic alkalo-sis, one would expect to find increased pH, increased plasma HCO3-, and increased PCO2.
Complex Acid-Base Disorders and Use of the Acid-Base Nomogram for Diagnosis
In some instances, acid-base disorders are not accom-panied by appropriate compensatory responses. When this occurs, the abnormality is referred to as a mixedacid-base disorder. This means that there are two ormore underlying causes for the acid-base disturbance. For example, a patient with low pH would be catego-rized as acidotic. If the disorder was metabolically medi-ated, this would also be accompanied by a low plasma HCO3– concentration and, after appropriate respiratory compensation, a low PCO2. However, if the low plasma pH and low HCO3– concentration are associated with elevated PCO2, one would suspect a respiratory compo-nent to the acidosis as well as a metabolic component. Therefore, this disorder would be categorized as a mixed acidosis. This could occur, for example, in a patient with acute HCO3– loss from the gastrointestinal tract because of diarrhea (metabolic acidosis) who also has emphysema (respiratory acidosis).
A convenient way to diagnose acid-base disorders is to use an acid-base nomogram, as shown in Figure 30–11. This diagram can be used to determine the type of acidosis or alkalosis, as well as its severity. In this acid-base diagram, pH, HCO3– concentration, and PCO2 values intersect according to the Henderson-Hassel-balch equation. The central open circle shows normal values and the deviations that can still be considered within the normal range. The shaded areas of the diagram show the 95 per cent confidence limits for the normal compensations to simple metabolic and respira-tory disorders.
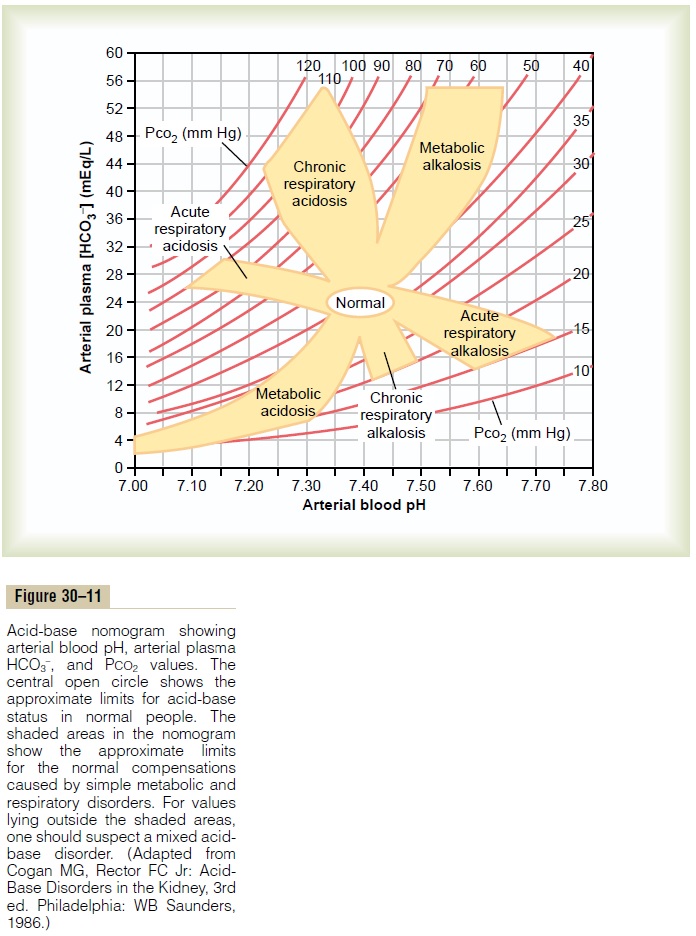
When using this diagram, one must assume that suf-ficient time has elapsed for a full compensatory response, which is 6 to 12 hours for the ventilatory com-pensations in primary metabolic disorders and 3 to 5 days for the metabolic compensations in primary respi-ratory disorders. If a value is within the shaded area, this suggests that there is a simple acid-base disturbance. Conversely, if the values for pH, bicarbonate, or PCO2 lie outside the shaded area, this suggests that there may be a mixed acid-base disorder.
It is important to recognize that an acid-base value within the shaded area does not always mean that there is a simple acid-base disorder. With this reservation in mind, the acid-base diagrams can be used as a quick means of determining the specific type and severity of an acid-base disorder.
For example, assume that the arterial plasma from a patient yields the following values: pH 7.30, plasma HCO3– concentration 12.0 mEq/L, and plasma PCO2 25 mm Hg. With these values, one can look at the diagram and find that this represents a simple metabolic acidosis, with appropriate respiratory compensation that reduces the PCO2 from its normal value of 40 mm Hg to 25 mm Hg.
A second example would be a patient with the fol-lowing values: pH 7.15, plasma HCO3– concentration 7 mEq/L, and plasma PCO2 50 mm Hg. In this example, the patient is acidotic, and there appears to be a meta-bolic component because the plasma HCO3– concentra-tion is lower than the normal value of 24 mEq/L. However, the respiratory compensation that would normally reduce PCO2 is absent, and PCO2is slightly increased above the normal value of 40 mm Hg. This is consistent with a mixed acid-base disturbance con-sisting of metabolic acidosis as well as a respiratory component.
The acid-base diagram serves as a quick way to assess the type and severity of disorders that may be con-tributing to abnormal pH, PCO2 , and plasma bicarbon-ate concentrations. In a clinical setting, the patient’s history and other physical findings also provide impor-tant clues concerning causes and treatment of the acid-base disorders.
Use of Anion Gap to Diagnose Acid-Base Disorders
The concentrations of anions and cations in plasma must be equal to maintain electrical neutrality. There-fore, there is no real “anion gap” in the plasma. However, only certain cations and anions are routinely measured in the clinical laboratory. The cation normally measured is Na+, and the anions are usually Cl– and HCO3–. The “anion gap” (which is only a diagnostic concept) is the difference between unmeasured anions and unmeasured cations, and is estimated as
Plasma anion gap = [Na+] – [HCO3–] – [Cl–]
= 144 – 24 – 108 = 10 mEq/L
The anion gap will increase if unmeasured anions rise or if unmeasured cations fall. The most important unmeasured cations include calcium, magnesium, and potassium, and the major unmeasured anions are albumin, phosphate, sulfate, and other organic anions. Usually the unmeasured anions exceed the unmeasured cations, and the anion gap ranges between 8 and 16 mEq/L.
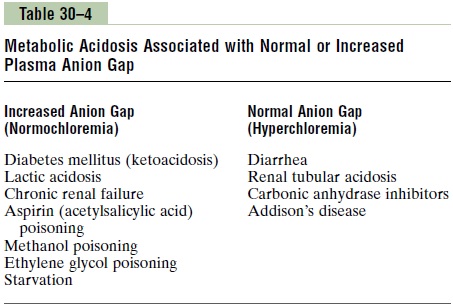
The plasma anion gap is used mainly in diagnosing different causes of metabolic acidosis. In metabolic acidosis, the plasma HCO3– is reduced. If the plasma sodium concentration is unchanged, the concentration of anions (either Cl– or an unmeasured anion) must increase to maintain electroneutrality. If plasma Cl– increases in proportion to the fall in plasma HCO3–, the anion gap will remain normal, and this is often referred to as hyperchloremic metabolic acidosis.
If the decrease in plasma HCO3– is not accompanied by increased Cl–, there must be increased levels of unmeasured anions and therefore an increase in the cal-culated anion gap. Metabolic acidosis caused by excess nonvolatile acids (besides HCl), such as lactic acid or ketoacids, is associated with an increased plasma anion gap because the fall in HCO3– is not matched by an equal increase in Cl–. Some examples of metabolic aci-dosis associated with a normal or increased anion gap are shown in Table 30–4. By calculating the anion gap, one can narrow some of the potential causes of meta-bolic acidosis.
Related Topics