Chapter: Ophthalmology: Glaucoma
Primary Open Angle Glaucoma
Primary Glaucoma
Primary Open Angle Glaucoma
Definition
Primary open angle glaucoma begins in middle-aged
and elderly patients with minimal symptoms that progressively worsen. The angle
of the anterior cham-ber characteristically remains open throughout the
clinical course of the dis-order.
Epidemiology:
Primary open angle glaucoma isby far the most common formof glaucoma and
accounts for over 90% of adult glaucomas. The incidence ofthe disorder
significantly increases beyond the age of 40, reaching a peak between the ages
of 60 and 70. Its prevalence among 40-year-olds is 0.9% as compared to 4.7% among
patients over the age of 50.
There appears to be a genetic predisposition for primary open angle glau-coma. Over one-
third of glaucoma patients have relatives with the same dis-order.
Patients with a positive family history are at
greater risk of developing the disorder.
Etiology (See also physiology and pathophysiology of aqueous humor circu-lation):
The cause of primary open angle glaucoma is not known, although
it is known that drainage of the aqueous
humor is impeded. The primary lesion occurs in the neuroretinal tissue of
the optic nerve as compression neuropathy of the optic nerve.
Symptoms:
The majority of patients with primary open angle glaucoma donot
experience any subjective symptoms for years. However, a small number of
patients experience occasional unspecific
symptoms such as headache, a burning sensation in the eyes, or blurred or
decreased vision that the patient may attribute to lack of eyeglasses or
insufficient correction. The patient may also perceive rings of color around
light sources at night, which has tradition-ally been regarded as a symptom of
angle closure glaucoma.
Primary open angle glaucoma often does not
exhibit typical symptoms for years. Regular examination by an ophthalmologist
is crucial for early diagnosis.
Primary open angle glaucoma can be far
advanced before the patient notices an extensive visual field defect in one or
both eyes.
It is crucial to diagnose the disorder as
early as possible because the prog-nosis for glaucoma detected in its early
stages is far better than for advanced glaucoma. Where increased intraocular
pressure remains undiagnosed or untreated for years, glaucomatous optic nerve
damage and the associated visual field defect will increase to the point of
blindness.
Diagnostic considerations:
Measurement of
intraocular pressure.Elevatedintraocular pressure in a routine ophthalmic examination
is an alarming sign.
Twenty-four-hour pressure curve.Fluctuations in intraocular pressure ofover 5 – 6 mm Hg may
occur over a 24-hour period.
Gonioscopy.The angle of the anterior chamber is open and appears as
normalas the angle in patients without glaucoma.
Ophthalmoscopy.Examination of the optic nerve reveals whether
glaucoma-tous cupping has already occurred and how far advanced the glaucoma
is. Where the optic disk and visual field are normal, ophthalmoscopic
examina-tion of the posterior pole under green light may reveal fascicular
nerve fiber defects as early abnormal findings.
Perimetry.Noise field perimetry is suitable as ascreening testas it makes thepatient aware of scotomas and makes it
possible to detect and describe them. The patient is shown a flickering monitor
displaying what resembles image noise on a television set. The patient will not
see the flickering points in the region of the scotoma. After this test, the
defect should be quantified by more specific methods. Automatic grid perimetry
is suitable for the early stages of
glaucoma. Special programs (such as the G1 program on the Octopus perime-ter
and the 30 – 2 program on the Humphrey perimeter devices) reveal the earliest
glaucomatous changes. In advanced
glaucoma, kinetic hand pe-rimetry with the Goldmann perimeter device is a
useful preliminary exami-nation to evaluate the remaining field of vision.
Differential diagnosis:
Two disorders are important in this context:
Ocular hypertension.Patients with ocular hypertension have significantlyincreased
intraocular pressure over a period of years without signs of glauco-matous
optic nerve damage or visual field defects. Some patients in this group will
continue to have elevated intraocular pressure but will not develop
glaucomatous lesions; the others will develop primary open angle glaucoma. The
probability that a patient will develop definitive glaucoma increases the
higher the intraocular pressure, the younger the patient, and the more
com-pelling the evidence of a history of glaucoma in the family.
Low-tension glaucoma.Patients with low-tension glaucoma exhibit typicalprogressive
glaucomatous changes in the optic disk and visual field without elevated
intraocular pressure. These patients are very difficult to treat because
management cannot focus on the control of intraocular pressure. Often these
patients will have a history of hemodynamic crises such as gastrointestinal or
uterine bleeding with significant loss of blood, low blood pressure, and
peripheral vascular spasms (cold hands and feet). Patients with glaucoma may
also experience further worsening of the visual field due to a drop in blood
pressure.
Caution should be exercised when using
cardiovascular and anti-hyper-tension medications in patients with glaucoma.
Treatment:
Indications for initiating treatment.
❖ Glaucomatous changes in the optic cup: Medical treatment should beinitiated where
there are signs of glaucomatous changes in the optic cup or where there is a
difference of more than 20% between the optic cups of the two eyes.
❖ Anyintraocular pressure exceeding 30 mm Hg
should be treated.
❖ Increasing glaucomatous
changes in the optic cup or increasing visual field defects: Regardless of the pressure measured, these
changes show that thecurrent pressure level is too high for the optic nerve and
that additional medical therapy is indicated. This also applies to patients
with advanced glaucomatous damage and threshold pressure levels (around 22 mm
Hg). The strongest possible medications are indicated in these cases to lower
pressure as much as possible (10 – 12 mm Hg).
❖Early stages: It is often difficult to determine whether therapy is
indicatedin the early stages, especially where intraocular pressure is elevated
slightly above threshold values. Patients with low-tension glaucoma exhibit
increasing cupping of the optical disk even at normal pressures (less than 22
mm Hg), whereas patients with elevated intraocular pressure (25 – 33 mm Hg) may
exhibit an unchanged optic nerve for years.
Patients with suspected glaucoma and risk
factors such as a family history of the disorder, middle myopia, glaucoma in
the other eye, or differences between the optic cup in the two eyes should be
monitored closely. Follow-up examinations should be performed three to four
times a year, especially for patients not undergoing treatment.
Medical therapy.
Available options in medical treatment of
glaucoma(see also Fig. 10.1):
❖ Inhibit aqueous humor production.
❖ Increase trabecular outflow.
❖ Increase uveoscleral outflow.
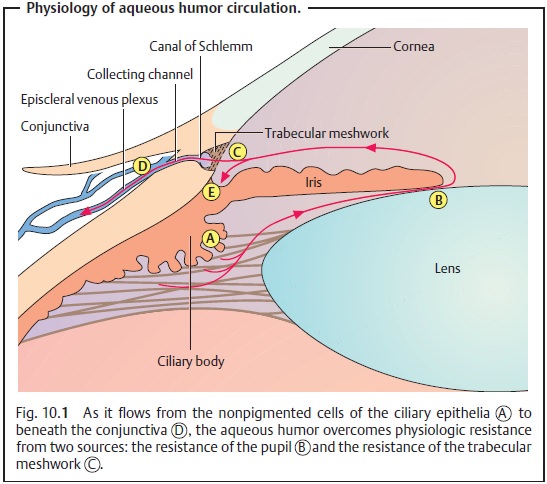
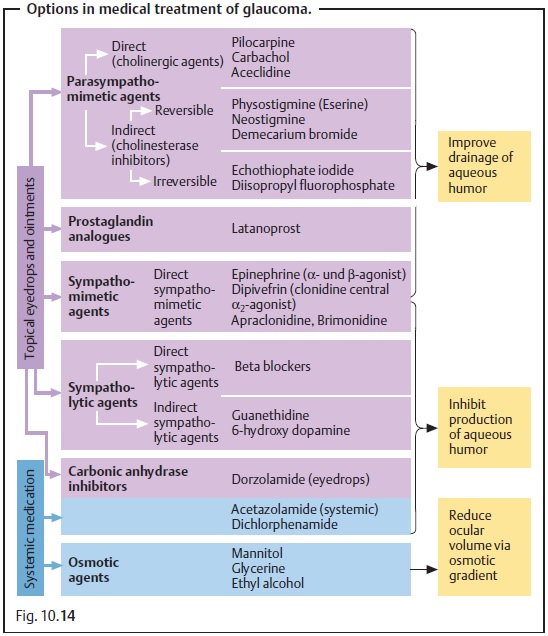
Fig. 10.14 and Table 10.3 list the various active ingredients and
substance groups available for medical treatment of glaucoma. For the sake of
completeness, Fig. 10.14 also lists traditional substances that are no longer used
today; these include substances that have too many side effects or have been
replaced by more efficient medications. Table 10.3 lists only those medications that are
actually used today.
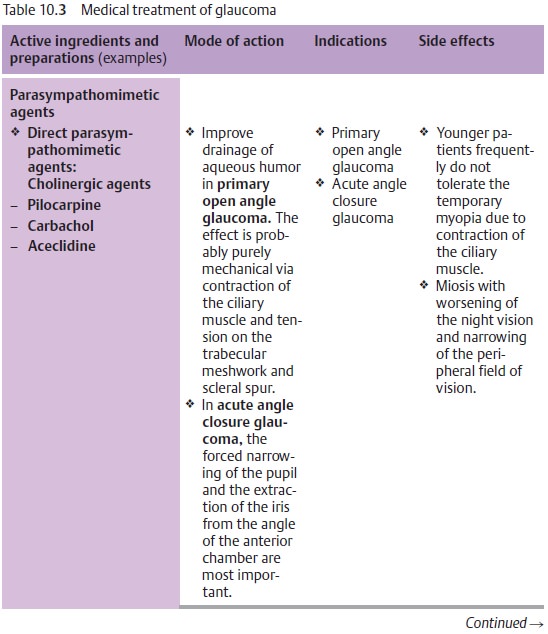
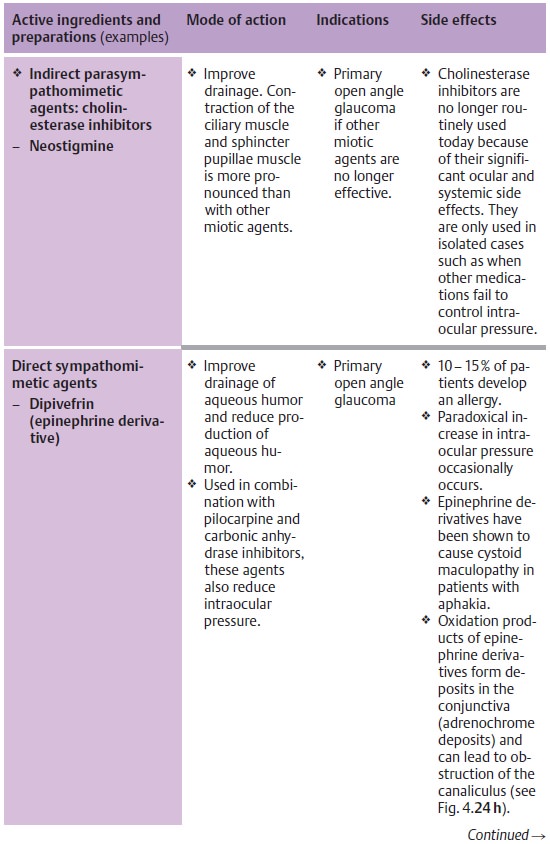
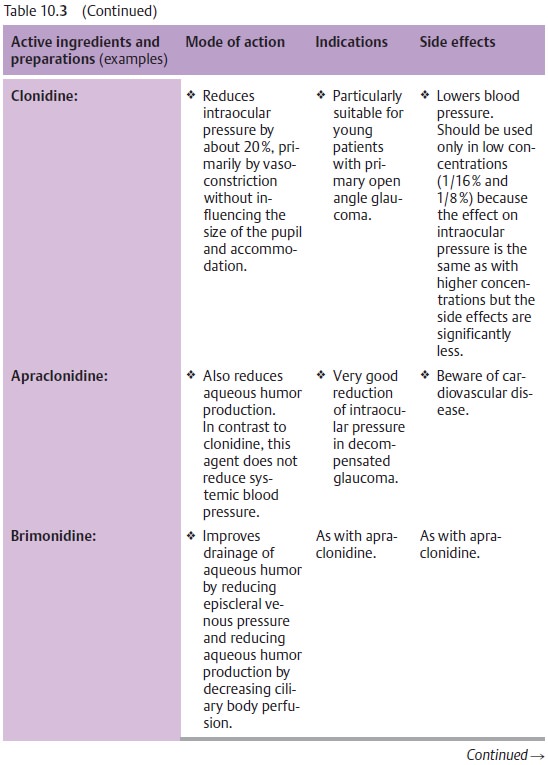
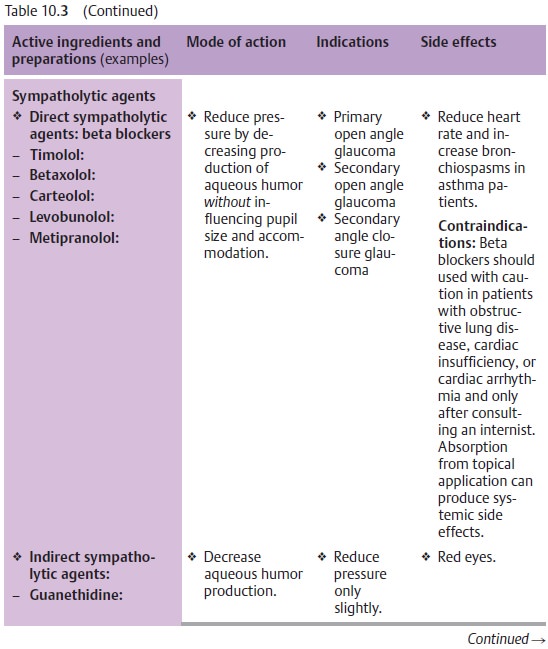
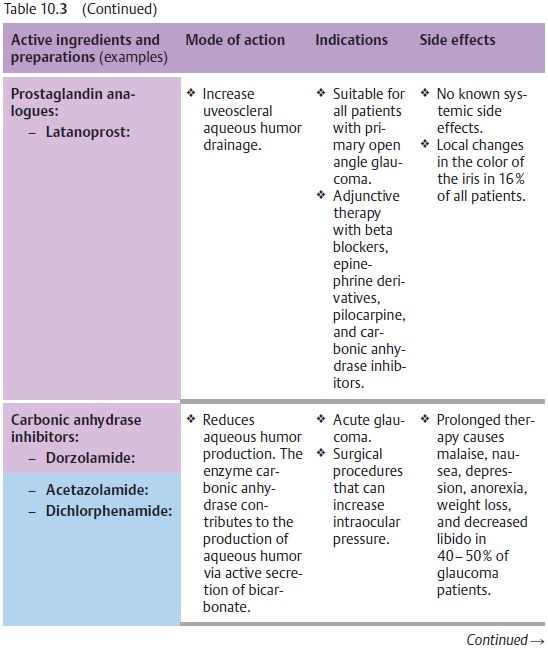
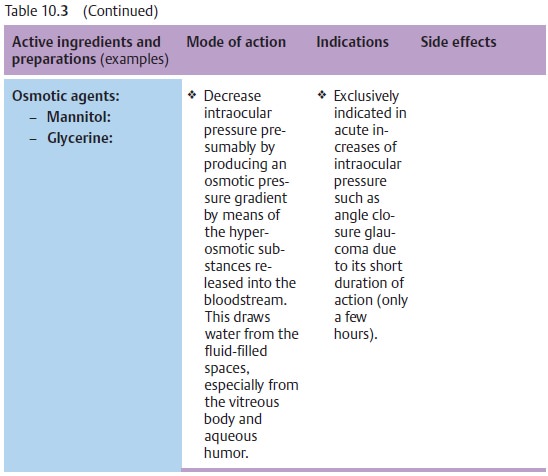
Principles of medical treatment of primary open angle glaucoma:
Medical therapy is the treatment of choice for
primary open angle glau-coma. Surgery is indicated only where medical therapy
fails.
There is no one generally applicable therapy
plan. However, several principles may
be formulated:
❖Where miosis is undesirable, therapy should
begin with beta blockers (Table 10.3).
❖Where miosis is not a problem (as is the case
with aphakia), therapy begins with miotic agents.
❖ Miotic agents may be supplemented with beta
blockers, epinephrine derivatives, guanethidine, dorzolamide and/or latanoprost
maximum topical therapy).
❖ Osmotic agents or carbonic anhydrase inhibitors (administered orally or intravenously) inhibit the production of aqueous humor. They can be administered temporarily in addition to topical medications. Their side effects usuallymake them unsuitable for prolonged treatment. The general rule is to try to use the weakest possible medications required to achieve normal pressure over a 24-hour period: as much as necessary, and as little as possible.
❖The effectiveness of any pressure-reducing
therapy should be verified by pressure analysis on the ward or on an outpatient
basis.
❖ The
effect of the eyedrops should not interfere with the patient’s ability to work.
Tolerance, effects, and side effects of the eyedrops should be repeatedly
verified on an individual basis during the course of treatment.
Surgical treatment of primary open angle glaucoma.
Indications:
❖ Medical therapy is insufficient.
❖The patient does not tolerate medical therapy.
Reactions include allergy, reduced vision due to narrowing of the pupil, pain,
and ciliary spasms, and ptosis.
❖The patient is not a suitable candidate for medical therapy due to lack of compliance or dexterity in applying eyedrops.
Argon laser trabeculoplasty:
❖ Principle: Laser burns in the trabecular
meshwork cause tissue contrac-tion that widens the intervening spaces and improves
outflow through the trabecular meshwork.
❖ Technique: Fifty to 100 focal laser burns are
placed in the anterior trabecu-lar meshwork (Fig. 10.15).
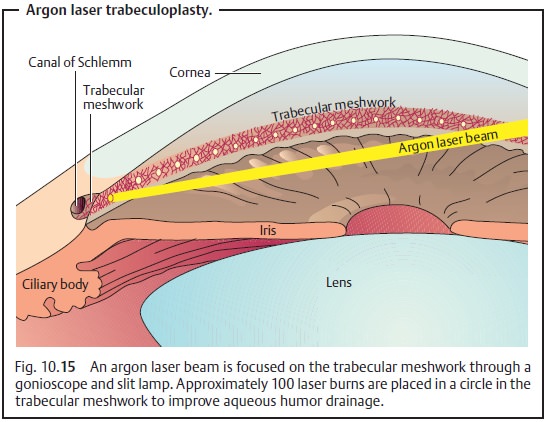
❖ Comment: Laser surgery in the angle of anterior chamber is possible only if the angle is open. The surgery itself is largely painless, may be performed as an outpatient procedure, and involves few possible complications. These may include bleeding from vascular structures near the angle and synechiae between the iris and individual laser burns. Argon laser trabeculoplasty can bring improvement with intraocular pressures up to 30 mm Hg. It decreases intraocular pressure by about 6 – 8 m Hg for about two years. Argon laser trabeculoplasty is only effective in about every sec-ond patient. The full effect occurs about four to six weeks postoperatively.
Filtration surgery:
❖ Principle: The aqueous humor is drained
through the anterior chamber through a subconjunctival scleral opening,
circumventing the trabecular meshwork. Formation of a thin-walled filtration
bleb is a sign of sufficient drainage of aqueous humor.
❖ Technique (Fig. 10.16a – c): First a conjunctival flap is raised, which may be either
fornix-based or limbal-based. Then a partial-thickness scleral flap is raised.
Access to the anterior chamber is gained via a goniotomy performed with a 1.5 mm trephine at the sclerocorneal
junction or via a rectangular trabeculectomy
performed with a scalpel and dissecting scissors. A periph-eral iridectomy
is then performed through this opening. The scleral flap is then loosely closed
and covered with conjunctiva.
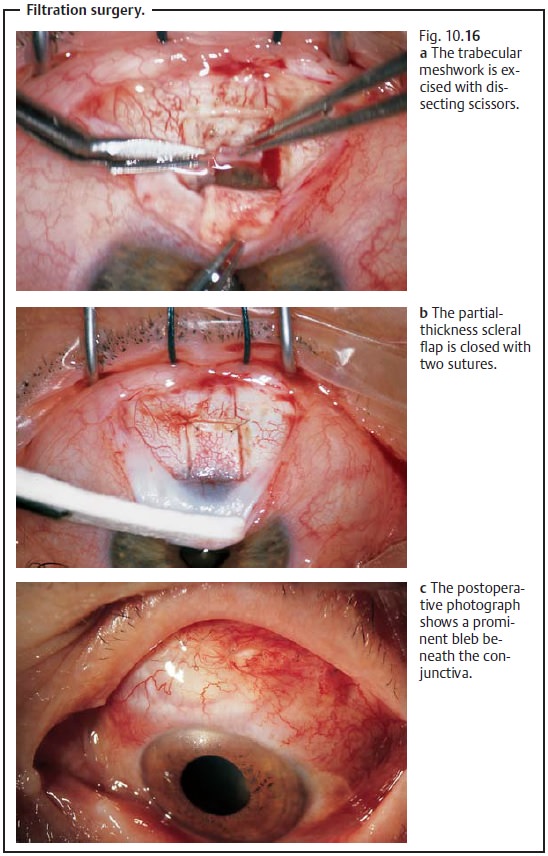
❖ Comment: A permanent reduction in intraocular
pressure is achieved in 80 – 85% of these operations.
Cyclodialysis:
❖ Principle: The aqueous humor is drained
through an opening into the suprachoroidal space.
❖
Technique: A full-thickness scleral incision is made down to the ciliary
body 4 mm posterior to the limbus. The sclera is then separated from the
ciliary body with a retractor and retracted anteriorly into the anterior
chamber. The ciliary body atrophies in the area of the incision, which also
helps to decrease the production of aqueous humor.
❖ Comment: This procedure is less common today
than it was in the 1980 s. One reason for this is that it is difficult to gauge
accurately the atrophy to the ciliary body. Occasionally severe hypotonia of
the globe will result, which then requires surgical intervention to close the
dialysis opening.
Cycloablation:
❖ Principle: Atrophy is induced in portions of the ciliary body through the intact sclera to reduce intraocular pressure by decreasing the amount of tissue producing aqueous humor.
❖
Technique:
– Cyclocryotherapy:
A cryoprobe is used to freeze the ciliary body at several points through the
sclera. This procedure can be repeated if nec-essary; the interventions have a
cumulative effect.
– Cyclodiathermy:
This method is similar to cyclocryotherapy except that a diathermy needle is
advanced through the sclera into the ciliary body to cauterize it with heat.
The procedure may be performed with or without prior dissection of a
partial-thickness scleral flap.
– Laser
cycloablation induces atrophy in the ciliary body using YAG laser or
high-energy diode laser pulses.
– Ultrasound
disruption induces atrophy in the ciliary body with high-frequency
ultrasound waves. These last two forms of therapy have been developed to induce
atrophy more effectively, more accurately, and in more controlled doses, which
is less traumatic for the eye.
❖ Comment: All these forms of cycloablation are
irreversible and cause per-manent hypotonia. Therefore, they represent the last
line of treatment options.
Prophylaxis:
No prophylactic action can be taken to prevent primary openangle
glaucoma.
Early diagnosis is crucial and can only be
made by an ophthalmologist. By the age of 40 at the latest, patients should
have their intraocular pressure measured regularly. The ophthalmologist
performs regular glaucoma screening examinations of intraocular pressure and pupil.
Therefore, the first pair of reading eyeglasses should always be pre-scribed by
an ophthalmologist.
Prognosis:
The prognosis depends greatly on the stage at
which primaryopen angle glaucoma is diagnosed. As a general rule, therapy is
more effective the earlier it can be initiated.
Related Topics