Chapter: Plant Biochemistry: A large diversity of isoprenoids has multiple functions in plant metabolism
Prenyl transferases catalyze the association of isoprene units
Prenyl transferases catalyze the association of isoprene units
Dimethylallyl pyrophosphate, which is formed by isomerization of isopen-tenyl pyrophosphate, is the acceptor for successive transfers of isopente-nyl moieties (Fig. 17.4). With the liberation of the pyrophosphate residue, dimethylallyl-PP condenses with isopentenyl-PP to produce geranyl-PP. In an analogous way, chain elongation is attained by further head-to-tail con-densations with isopentenyl-PP, and so farnesyl-PP and geranylgeranyl-PP are formed one after the other.
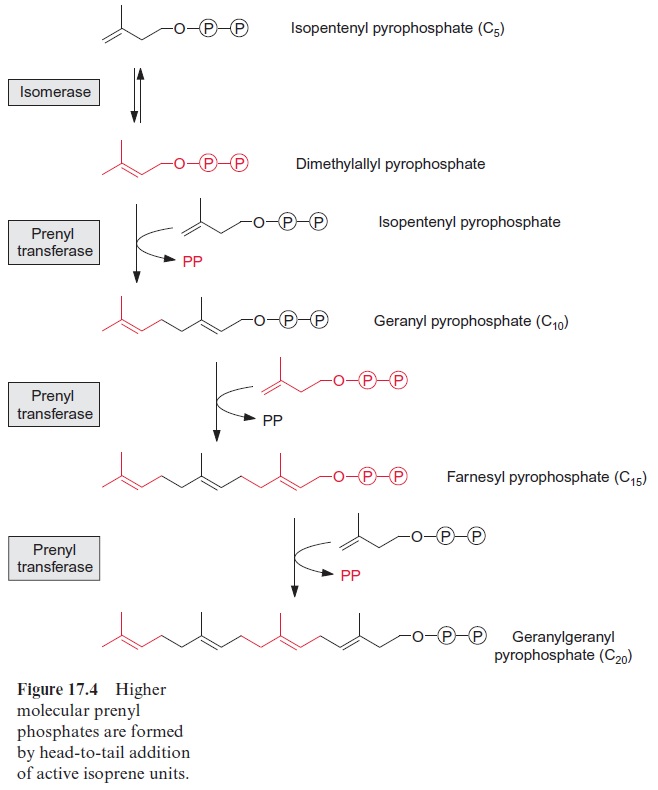
The transfer of the isopentenyl moieties is catalyzed by prenyl transferases. Prenyl residues are a collective term for isoprene or polyisoprene residues. A special prenyl transferase is required for the production of each of the pre-nyl pyrophosphates mentioned. For example, the prenyl transferase termed geranyl-PP synthase catalyzes only the synthesis of geranyl-PP. However, farnesyl-PP synthase synthesizes farnesyl-PP in two discrete steps: from dimethylallyl-PP and isopentenyl-PP, first geranyl-PP is formed, but this inter-mediate remains bound to the enzyme and reacts further with another isopen-tenyl-PP to produce farnesyl-PP. Analogously,geranylgeranyl-PP synthase catalyzes all three steps of the formation of geranylgeranyl-PP. Table 17.1 shows that each of these prenyl pyrophosphates is the precursor for the synthesis of structurally and functionally specific isoprenoids, including hemiterpenes, monoterpenes, and sesquiterpenes. As these prenyl pyrophos-phates are synthesized by different enzymes, the synthesis of a certain prenyl pyrophosphate can be regulated by induction or repression of the corre-sponding enzyme. It appears that there is a synthesis pathway from isopente-nyl pyrophosphate to geranylgeranyl pyrophosphate, not only in the cytosol but also in the plastids. The differences between these two pathways have not yet been resolved in detail.
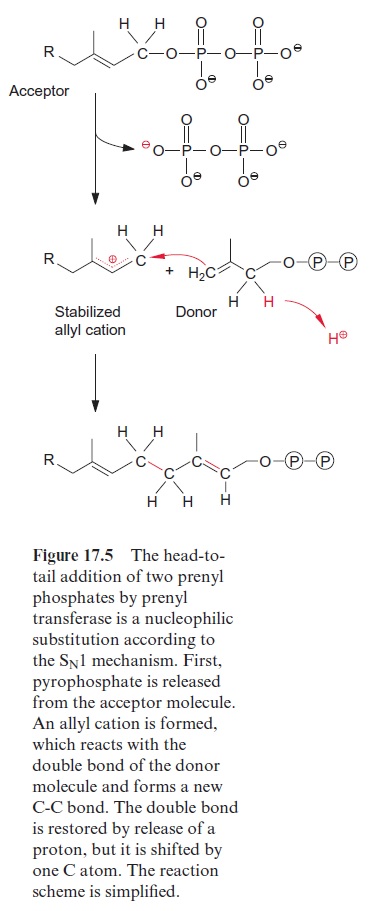
The formation of a C-C linkage between two isoprenes proceeds by nucle-ophilic substitution (Fig. 17.5): an Mg++ ion, bound to the prenyl trans-ferase, facilitates the release of the negatively charged pyrophosphate residue from the acceptor molecule, whereby a positive charge remains at the termi-nal C atom (C-1), which is stabilized by the neighboring double bond. The allyl cation thus formed reacts with the terminal C-C double bond of the donor molecule and a new C-C bond is formed with the release of a proton. According to the same reaction mechanism, not only isoprene chains, but also rings are formed, leading to the exceptional diversity of isoprenoids.
Related Topics