Chapter: Plant Biochemistry: A large diversity of isoprenoids has multiple functions in plant metabolism
Higher plants have two different synthesis pathways for isoprenoids
Higher plants have two different synthesis pathways for isoprenoids
Precursor for the synthesis of isoprenoids is isopentenyl pyrophosphate. Its synthesis proceeds in higher plants and some groups of algae in two different ways, one located in the cytosol and the other in the plastids.
Acetyl CoA is a precursor for the synthesis of isoprenoids in the cytosol
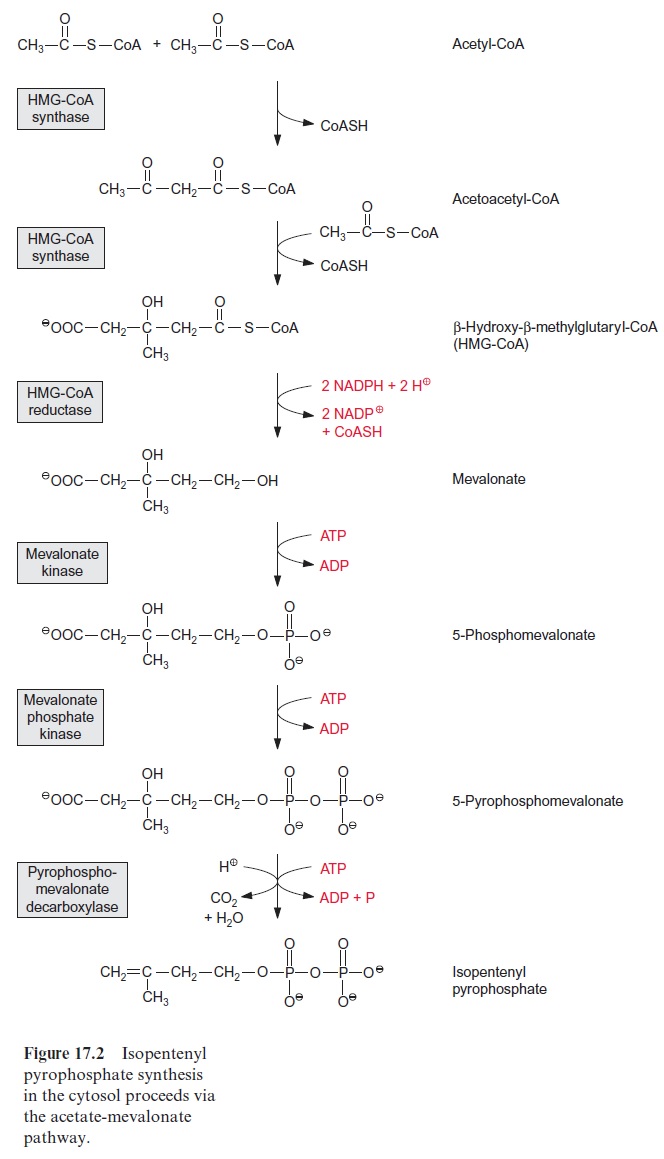
The basis for the elucidation of this isoprenoid biosynthesis pathway was the discovery by Konrad Bloch (USA, likewise a joint winner of the Nobel Prize in Medicine in 1964) that acetyl CoA is a precursor for the biosyn-thesis of steroids. Figure 17.2 shows the synthesis of the intermediary product isopentenyl pyrophosphate: two molecules of acetyl CoA react to produce acetoacetyl CoA and then with another acetyl CoA yielding β-hydroxy- β-methylglutaryl CoA (HMG CoA). In yeast and animals, these reactions are catalyzed by two different enzymes, whereas in plants a sin-gle enzyme, HMG CoA synthase, catalyzes both reactions. The esterified carboxyl group of HMG CoA is reduced by two molecules of NADPH to a hydroxyl group, accompanied by hydrolysis of the energy-rich thioester bond. Thus mevalonate is formed in an irreversible reaction. The forma-tion of mevalonate from HMG CoA is an important regulatory step of iso-prenoid synthesis in animals. It has not yet been resolved whether this also applies to plants. A pyrophosphate ester is formed in two successive phos-phorylation steps, catalyzed by two different kinases. With consumption of a third molecule of ATP, involving the transitory formation of a phosphate ester, a carbon-carbon double bond is generated and the remaining car-boxyl group is removed. Isopentenyl pyrophosphate thus formed is the basic element for the formation of an isoprenoid chain. This synthesis of isopentenyl pyrophosphate, termed the acetate-mevalonate pathway, is located in the cytosol. It is responsible for the synthesis of sterols, certain sesquiterpenes, and the side chain of ubiquinone.
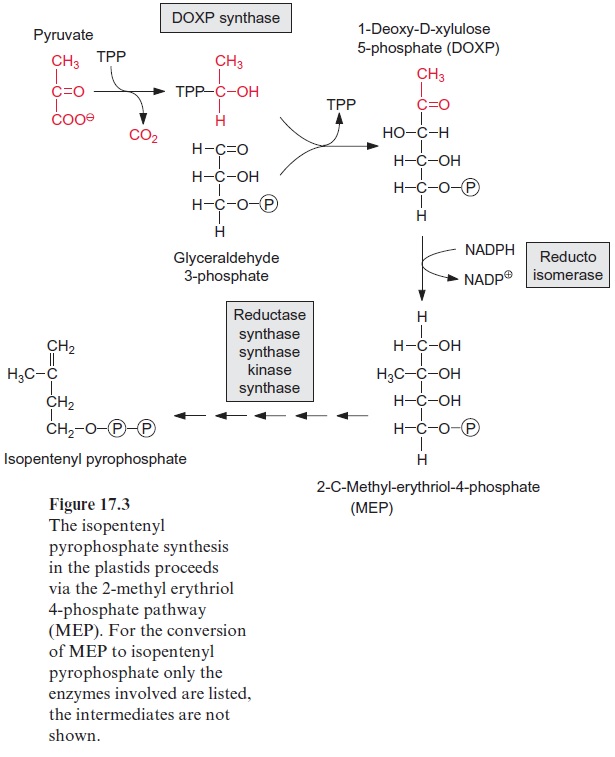
Pyruvate and D-glyceraldehyde-3-phosphate are the precursors for the synthesis of isopentyl pyrophosphate in plastids
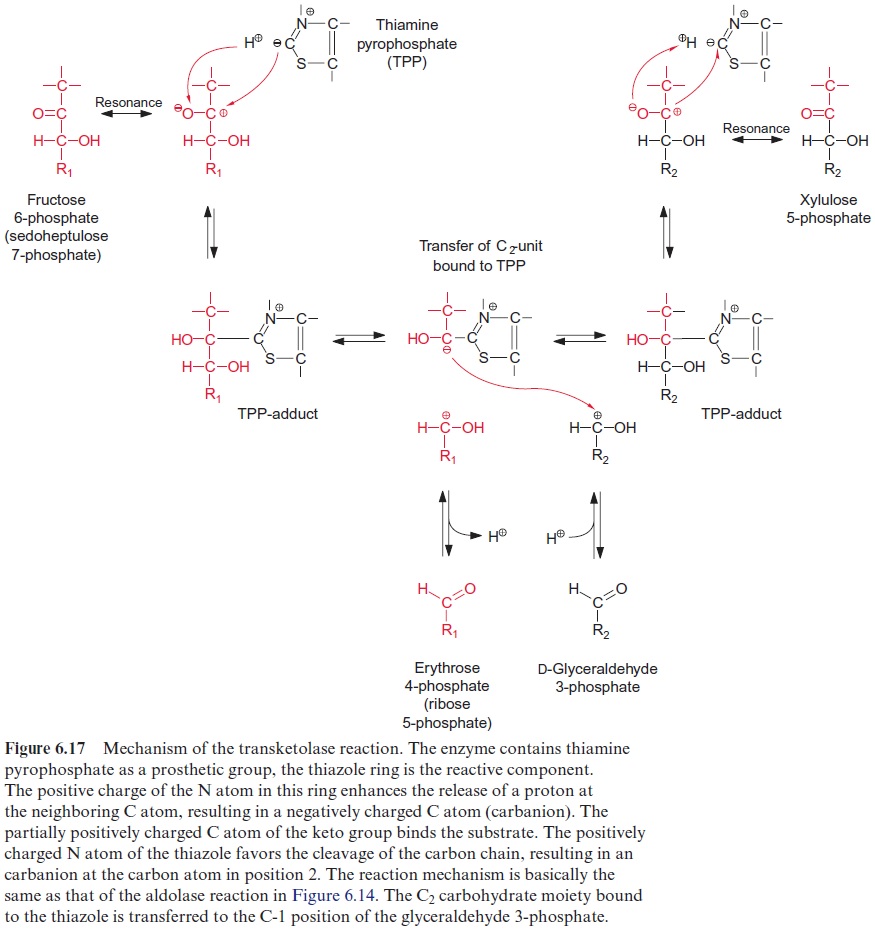
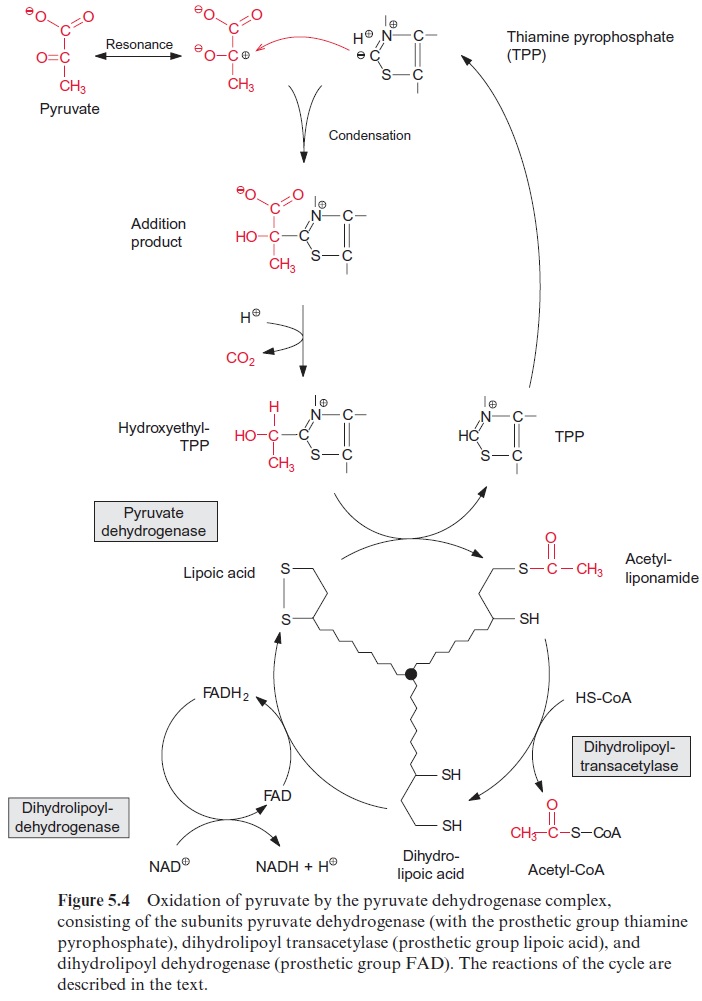
The acetate-mevalonate pathway can be blocked by mevilonin, a very spe-cific inhibitor of HMG CoA reductase. Experiments with plants showed that mevilonin inhibits the isoprenoid synthesis in the cytosol, but not in the plas-tids. These findings led to the discovery that the synthesis of isopentenyl pyro-phosphate in the plastids follows a different pathway from that in the cytosol (Fig. 17.3). For the plastidal synthesis pathway, the precursors are pyruvate and D-glyceraldehyde-3-phosphate. As in the pyruvate dehydrogenase reac-tion (Fig. 5.4), pyruvate is decarboxylated via thiamine pyrophosphate (TPP), and then, as in the transketolase reaction (Fig. 6.17), is transferred to D-glyceraldehyde-3-phosphate to yield 1-deoxy-D-xylulose-5-phosphate (DOXP). After isomerization and reduction by NADPH, 2-C-methyl-D-erythritol-4-phosphate (MEP) is synthesized. MEP is then activated by react-ing with CTP to yield CDP methyl erythriol. Two further reduction steps, followed by dehydration and phosphorylation, finally yield isopentenyl pyro-phosphate. The MEP-synthase pathway for isoprenoids is present in bacte-ria, algae, and plants, but not in animals. A large part of plant isoprenoids, including the hemiterpene isoprene, monoterpenes like pinene and limonene, diterpenes (e.g., phytol chains, gibberellin, abietic acid as oleoresin constitu-ent) as well as tetraterpenes (carotenoids) are synthesized via the MEP syn-thase pathway located in the plastids. Also, the side chains of chlorophyll and plastoquinone are synthesized by this pathway. Sesquiterpenes and triterpe-nes, on the other hand, according to our present knowledge are synthesized by the mevalonate pathway in the cytosol (Fig. 17.2).
Related Topics