Chapter: Plant Biochemistry: A large diversity of isoprenoids has multiple functions in plant metabolism
Many aromatic compounds derive from geranyl pyrophosphate
Many aromatic compounds derive from geranyl pyrophosphate
The monoterpenes comprise a large number of open chain and cyclic iso-prenoids, many of which, due to their high volatility and their lipid character, are classified as essential oils. Many of them have a distinctive, often pleasant odor and are, for example, responsible for the typical scents of pine needles, thyme, lavender, roses, and lily of the valley. Flower scents attract insects for the distribution of pollen, but in addition some vola-tiles also repel insects and other animals and thus protect the plants from herbivores.
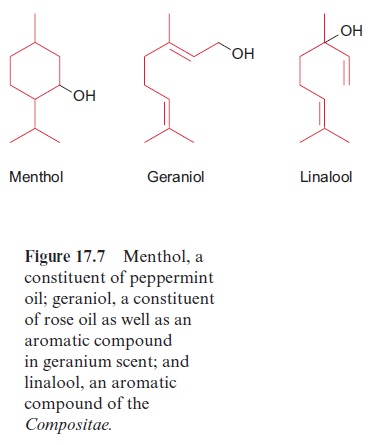
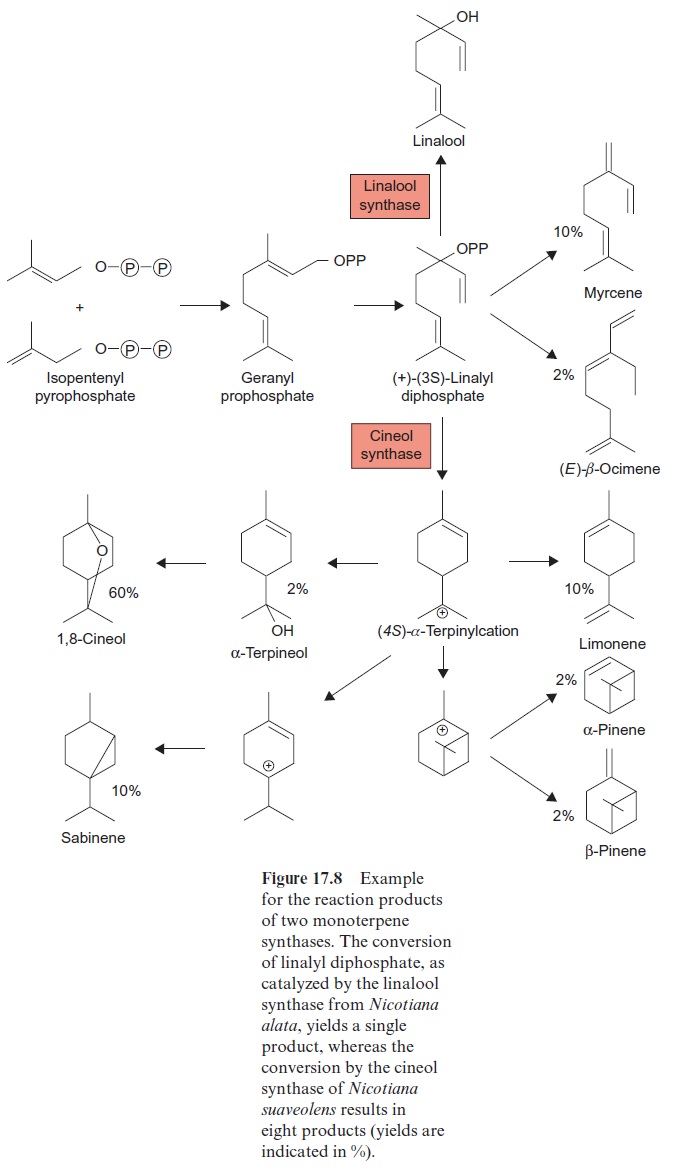
The hydrolysis of geranyl-PP results in the formation of the alcohol geraniol (Fig. 17.7), the main constituent of rose oil. Geraniol has the typi-cal scent of freshly cut geraniums. Geranyl-PP is a precursor for the synthe-sis of monoterpenes via monoterpene synthases. These enzymes belong to a common enzyme family, which typically possess characteristic sequence motifs and similar active centers, and produce a great variety of products. Figure 17.8 shows the products of two closely related monoterpene synthases. Whereas the linalool synthase from Nicotiana alata produces only one product linalool, the cineol synthase from Nicotiana suaveolens, as a multiproduct enzyme, yields eight products (60% cineol, 10% each myrcene, limonene and sabinene and 2% each ocimen, terpineol,α-pinene and β-pinene). Thus a variety of compounds can be synthesized by a single enzyme. Monoterpenes occur as scents in flowers to lure insects, but they are also contained in plants as insect repellent. The monoterpenes myrcene, limonene, α-pinene and β-pinene are major constituents of the resin (termed olioresin) of conifers. They are toxic for many insects and thus act as a protection against herbi vores. Conifers respond to an attack by bark beetles with a strong increase of cyclase activity, which results in enhanced formation of cyclic monoterpenes, e.g., pinene, limonene. Limonene is also found in the leaves and peel of lem-ons. Another example of a monoterpene is menthol (Fig. 17.7), the main constituent of peppermint oil. It serves the plant as an insect repellent. Many other monoterpenes containing carbonyl and carboxyl groups can be synthe-sized by plants, which are not discussed here.
Related Topics