Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Individuals, populations, and assemblages
Population dynamics and regulation - Diversity of Fishes
Populations
Simply defined, a population consists of all the individuals of a particular species in a given area. Because populations form the matrix in which individual survival and reproduction occur, expanded definitions recognize the importance of genetic structure: a population is therefore “a gene pool that has continuity through time because of the reproductive activities of the individuals in the population” (Wootton 1990, p. 280). Populations grow and shrink in numbers as a result of the actions and interactions of their individuals, which can change relative gene frequencies of the population. Much of ecology has been devoted to describing, understanding, and predicting the nature and causes of population numerical growth and decline and of genetic structure (the effects of competition and predation on population size are discussed in the context of “Assemblages” below).
Population dynamics and regulation
Population size changes as a function of four major processes: birth, death, immigration, and emigration. Birth and death rates (age-specific reproduction andsurvivorship rates of individuals) can be used to calculate the approx.
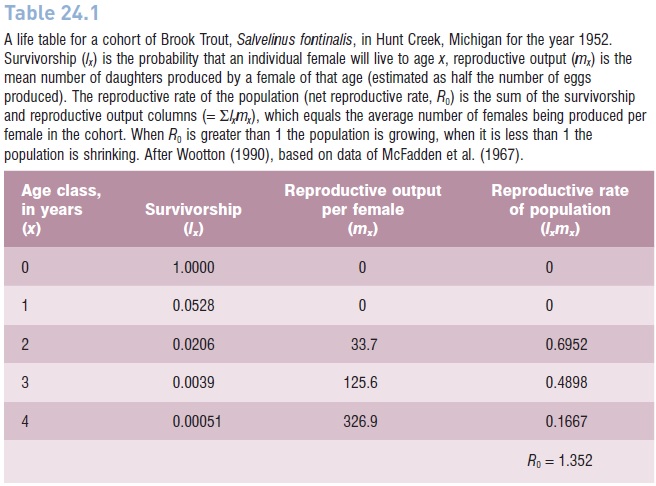
Table 24.1
A life table for a cohort of Brook Trout, Salvelinus fontinalis, in Hunt Creek, Michigan for the year 1952. Survivorship (lx) is the probability that an individual female will live to age x, reproductive output (mx) is the mean number of daughters produced by a female of that age (estimated as half the number of eggs produced). The reproductive rate of the population (net reproductive rate, R0) is the sum of the survivorship and reproductive output columns (=lxmx), which equals the average number of females being produced per female in the cohort. When R0 is greater than 1 the population is growing, when it is less than 1 the population is shrinking. After Wootton (1990), based on data of McFadden et al. (1967).
Migration, both in and out of a population, greatly complicates any attempt at predicting future population size. Fish populations increase in size due to migration as a result of either recruitment or colonization. Recruitment usually refers to the addition to the population through reproduction, as when larvae settle out of the plankton and into the population. In fisheries terminology, recruitment generally refers to the addition of potentially catchable individuals to the stock in question, stock being essentially synonymous with population. Colonization is the addition by movement of established individuals between habitats, such as when juveniles move from a nursery habitat to an adult habitat.
Because many fishes export their reproductive products in the form of pelagic larvae that are dispersed widely, reproductive events in a particular population may have little effect on later local population size. As a result, it was widely held that, for most marine populations, minimal correspondence existed between stock and recruitment (the stock : recruitment relationship). Minimal relationship meant that current population size was not a reliable predictor of future population size, suggesting that intense local fishing did not necessarily drive down future stocks (e.g., Rothschild 1986). However, more recent analyses suggest that a positive relationship does exist between stock and recruitment in many fisheries, in part perhaps because “export” of larvae from parental habitat is not as general as traditionally thought (e.g., Sponaugle et al. 2002; Gerlach et al. 2007). Myers and Barrowman (1996) found a generally positive stock : recruitment relationship in 83 mostly marine species indicating that (i) higher recruitment occurred when spawner abundance was high; (ii) lower recruitment occurred when abundance was low; and (iii) populations below the median abundance level had lower recruitment than populations above it. Specific examples included such well-known, depleted fisheries as Bluefin Tuna, Atlantic Cod, and large sharks. Life tables are therefore useful not only in relatively closed populations, such as in ponds and lakes, but also in many commercially important marine species.
Regardless of locale, fish populations vary widely and notoriously in size. The concept of the year class or cohort is important in understanding these dynamics. High population density may not necessarily indicate a sustainably reproducing population because many of the individuals in that population may come from a single year class, whereas most other years may have seen little successful reproduction (Fig. 24.2). If the successful year class is approaching the usual maximum age for that species and no younger year class is abundant, overexploitation of the dominant year class can lead to very rapid population collapse. Year class strength then becomes a critical statistic in determining management schemes for exploited populations. The spectacular success of the campaign to restore Striped Bass, Morone saxatilis (Moronidae), to the Chesapeake Bay focused on protecting the 1982 year class until 95% of those females had matured (Ross 1997; Secor 2000).
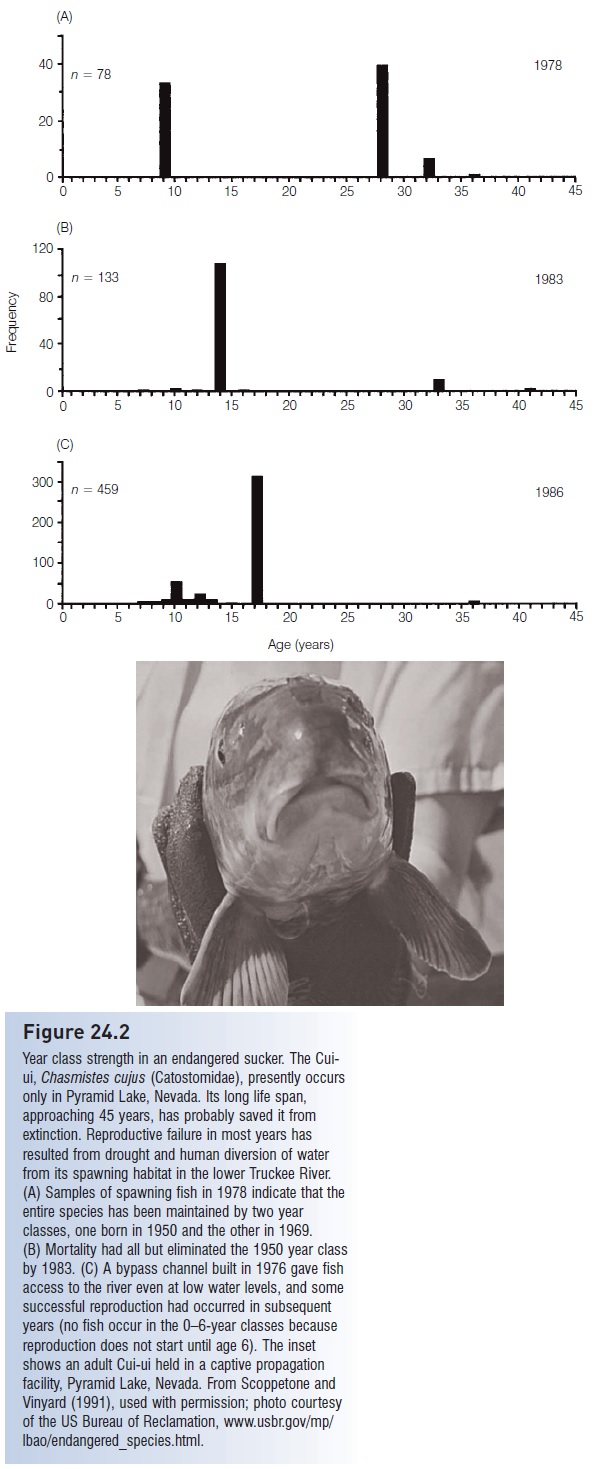
Figure 24.2
Year class strength in an endangered sucker. The Cuiui, Chasmistes cujus (Catostomidae), presently occurs only in Pyramid Lake, Nevada. Its long life span, approaching 45 years, has probably saved it from extinction. Reproductive failure in most years has resulted from drought and human diversion of water from its spawning habitat in the lower Truckee River. (A) Samples of spawning fish in 1978 indicate that the entire species has been maintained by two year classes, one born in 1950 and the other in 1969. (B) Mortality had all but eliminated the 1950 year class by 1983. (C) A bypass channel built in 1976 gave fish access to the river even at low water levels, and some successful reproduction had occurred in subsequent years (no fish occur in the 0–6-year classes because reproduction does not start until age 6). The inset shows an adult Cui-ui held in a captive propagation facility, Pyramid Lake, Nevada. From Scoppetone and Vinyard (1991), used with permission; photo courtesy of the US Bureau of Reclamation, www.usbr.gov/mp/lbao/endangered_species.html.
Variation in numbers among year classes points out another important feature of fish populations, which is that they are size structured. Indeterminate growth and over-lapping generations create a situation where a population may include individuals of very different sizes, differing in body mass by as much as four or more orders of magnitude (e.g., consider Bluefin Tuna that weigh a fraction of a gram at birth and grow to an adult size exceeding 500 kg in mass, a range of seven orders of magnitude). Size structuring can affect population regulation because multiple size classes provide the potential for intraspecific competition and cannibalism, which in turn may lead to differences in habitat and other resource use because of avoidance of one group by another.
Such intraspecific variation has led to the concept of the ontogenetic niche, which recognizes the very different ecological roles that different age and size conspecifics are likely to play in an assemblage (Werner & Gilliam 1984; Osenberg et al. 1992). For example, Pinfish (Lagodon rhomboides, Sparidae) start off as a carnivore and progressively shift to increasing herbivory in five distinct phases (Stoner & Livingston 1984). Largemouth Bass (Micropterus salmoides, Centrarchidae) initially feed on zooplankton, then on littoral invertebrates, and then finally on fish, including conspecifics. As juveniles, they compete with adult Bluegill (Lepomis macrochirus) for zooplankton. Young Largemouth are, however, miniature adults and are morphologically constructed as piscivores. They are consequently less efficient planktivores than are adult Bluegill, and this morphological constraint makes them inferior competitors (they even the score later when Bluegill become their major prey) (Werner & Gilliam 1984). At each size, a fish is likely to have a different set of competitors and predators, some overlapping with the previous set, producing an incredibly complex set of interactions within a community containing even a small number of species (e.g., Fig. 25.14).
Death can come at any time, but certain life history stages are more dangerous than others. Eggs and larvae are by far the most vulnerable periods (see Larval feeding and survival). Estimates of mortality for marine fish populations range from about 10% to 85% per day for eggs, from 5% to 70% per day for yolk-sac larvae, and from 5% to 55% per day for feeding larvae (Bailey & Houde 1989). These are daily rates. When compounded over the larval life of a species, the magnitude of the loss is more striking. For example, jack mackerel, Trachurus symmetricus, require 8 days from hatching until they resorb their yolk sac and begin independent feeding. During this time, when mortality falls from 80% to 50% per day, 99.5–99.9% of larvae are lost to predation. Exogenous feeding adds the hazard of starvation; during the first week after yolk-sac absorption, larvae die at a rate of 45% per day from starvation alone, to which can be added predation losses (Hewitt et al. 1985).
Most workers agree that predation is the major source of mortality for eggs and larvae and that mortality is strongest on eggs and small larvae. The list of predators on larvae is long and includes numerous invertebrates (ctenophores, siphonophores, jellyfishes, copepods, chaetognaths, euphausiids, shrimps, amphipods) as well as fishes. As fish grow, their strength, swimming speed, food getting ability, and general escape ability increase. Estimates of the mortality of juveniles and adults are diametrically different from the rates experienced by eggs and larvae, for example 99.9% daily survival for juvenile or adult nototheniid icefishes, English Sole, Winter Flounder, cutlassfishes (Trichiuridae), mackerel, and tuna (McGurk 1986; Richards & Lindeman 1987). Freshwater salmonids (Brown Trout, Brook Trout, Rainbow Trout, Coho Salmon) sustain relatively high annual mortality rates of 60–90% of the adult population, which is still less than the values experienced by eggs and larvae (Alexander 1979).
Cannibalism (intraspecific predation) is widespread in fishes and may play a dominant role in population regulation in some species (Dominey & Blumer 1984; Smith & Reay 1991; Elgar & Crespi 1992). Cannibalism occurs in many chondrichthyans and in at least 36 families of teleost fishes, including herbivores, scavengers, planktivores, and piscivores. Cannibalism can have a significant impact on population dynamics. Between 30% and 70% of egg consumption is caused by conspecifics among anchovies (Engraulidae) and whitefishes (Salmonidae). In addition, adults may eat larvae and juveniles, including their own offspring, and young fish may eat siblings as well as un - related individuals. Sixty percent of annual mortality in Walleye Pollock, Theragra chalcogramma (Gadidae), and 25% of mortality in Yellow Perch, Perca flavescens, has been attributed to cannibalism of juveniles. Year class strength is thought to be strongly dependent on cannibalism rates in Pike (Esocidae), Cod, Haddock, and Whiting (Gadidae), Walleye and Perch (Percidae), and Nile Perch (Latidae). In Lake Victoria Nile Perch, cannibalism is considered the major cause of perch mortality, with important consequences for assemblage structure and human welfare alike (see Introduced predators). In lakes where one or only a few species occur, cannibalism may bethe major population regulatory mechanism. In such situations, giant cannibal morphs that are specialized to feed on conspecifics may develop (e.g., landlocked Arctic Char, Salvelinus alpinus; Sparholt 1985; Riget et al. 1986) (Fig. 24.3); such cannibalistic polyphenism is also known among larval salamanders and frogs.

Figure 24.3
Four morphs of Arctic Char that differ anatomically, behaviorally, and ecologically can be found in a single lake. Shown here are adults of the four morphs from an Icelandic lake. They are, from top to bottom, the large, benthic feeding morph (33 cm long), the small benthic feeding morph (8 cm), the piscivorous morph (35 cm), and the plantivorous morph (19 cm). Photo by S. Skulason, from Skulason and Smith (1995), used with permission.
At first glance, cannibalism might appear counterproductive evolutionarily. However, as long as the cannibal is not eating close relatives, no fitness costs are incurred, aside from possible transmission of host-specific pathogens and parasites, whereas potential competitors are eliminated. More importantly, conspecifics represent a highly nutritious protein meal made up of optimum proportions of vitamins, minerals, and amino acids for the species in question, producing high growth rates (e.g., walleye, Walleye Pollock) and enhanced reproductive output (e.g., Mosquitofish, Poeciliidae). Even when kin are consumed, the benefits to the cannibal of reduced competition, increased growth, and enhanced reproduction could outweigh the current costs of losing a few relatives (Dominey & Blumer 1984; Smith & Reay 1991; Sogard & Olla 1994).
Related Topics