Chapter: Mechanical : Engineering materials and metallurgy : Non-Metallic Materials
Polymer
NON-METALLIC MATERIALS
Polymer
Appearance
of real linear polymer chains as recorded using an atomic force microscope on
surface under liquid medium. Chain contour length for this polymer is ~204 nm;
thickness is ~0.4 nm.
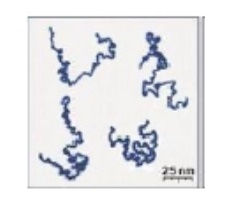
A polymer
is a large molecule (macromolecule) composed of repeating structural units.
These subunits are typically connected by covalent chemical bonds. Although the
term polymer is sometimes taken to
refer to plastics, it actually encompasses a large class of natural and synthetic materials with a wide
variety of properties.
Because of the extraordinary range of properties of
polymeric materials, they play an essential and ubiquitous role in everyday
life. This role ranges from familiar synthetic plastics and elastomers to
natural biopolymers such as nucleic acids and proteins that are essential for
life.
Natural polymeric materials such as shellac, amber,
and natural rubber have been used for centuries. A variety of other natural
polymers exist, such as cellulose, which is the main constituent of wood and
paper. The list of synthetic polymers includes synthetic rubber, Bakelite,
neoprene, nylon, PVC, polystyrene, polyethylene, polypropylene,
polyacrylonitrile, PVB, silicone, and many more.
Most commonly, the continuously linked backbone of
a polymer used for the preparation of plastics consists mainly of carbon atoms.
A simple example is polyethylene, whose repeating unit is based on ethylene
monomer. However, other structures do exist; for example, elements such as
silicon form familiar materials such as silicones, examples being silly putty
and waterproof plumbing sealant. Oxygen is also commonly present in polymer
backbones, such as those of polyethylene glycol, polysaccharides (in glycosidic
bonds), and DNA (in phosphodiester bonds).
Polymers are studied in the fields of polymer
chemistry, polymer physics, and polymer science
Etymology
The word polymer is derived from the Greek
words πολύ- - poly- meaning
"many"; and μέρος - meros meaning
"part". The term was coined in 1833 by Jöns Jacob Berzelius, although his definition of a polymer
was quite different from the modern definition.
Historical development
Starting in 1811, Henri Braconnot did pioneering
work in derivative cellulose compounds, perhaps the earliest important work in
polymer science. The development of vulcanization later in the nineteenth
century improved the durability of the natural polymer rubber, signifying the
first popularized semi-synthetic polymer. In 1907, Leo Baekeland created the
first completely synthetic polymer, Bakelite, by reacting phenol and
formaldehyde at precisely controlled temperature and pressure. Bakelite was
then publicly introduced in 1909.
Despite
significant advances in synthesis and characterization of polymers, a correct
understanding of polymer molecular structure did not emerge until the 1920s.
Before then, scientists believed that polymers were clusters of small molecules
(called colloids), without definite molecular weights, held together by an
unknown force, a concept known as association theory. In 1922, Hermann
Staudinger proposed that polymers consisted of long chains of atoms held
together by covalent bonds, an idea which did not gain wide acceptance for over
a decade and for which Staudinger was ultimately awarded the Nobel Prize. Work
by Wallace Carothers in the 1920s also demonstrated that polymers could be
synthesized rationally from their constituent monomers. An important
contribution to synthetic polymer science was made by the Italian chemist
Giulio Natta and the German chemist Karl Ziegler, who won the Nobel Prize in
Chemistry in 1963 for the development of the Ziegler-Natta catalyst. Further
recognition of the importance of polymers came with the award of the Nobel Prize
in Chemistry in 1974 to Paul Flory, whose extensive
work on polymers included the kinetics of
step-growth polymerization and of addition polymerization, chain transfer,
excluded volume, the Flory-Huggins solution theory, and the Flory convention.
Synthetic polymer materials such as nylon,
polyethylene, Teflon, and silicone have formed the basis for a burgeoning
polymer industry. These years have also shown significant developments in
rational polymer synthesis. Most commercially important polymers today are
entirely synthetic and produced in high volume on appropriately scaled organic
synthetic techniques. Synthetic polymers today find application in nearly every
industry and area of life. Polymers are widely used as adhesives and
lubricants, as well as structural components for products ranging from
children's toys to aircraft. They have been employed in a variety of biomedical
applications ranging from implantable devices to controlled drug delivery.
Polymers such as poly(methyl methacrylate) find application as photoresist
materials used in semiconductor manufacturing and low-k dielectrics for use in
high-performance microprocessors. Recently, polymers have also been employed as
flexible substrates in the development of organic light-emitting diodes for
electronic display.
Related Topics