Chapter: Mechanical : Engineering materials and metallurgy : Non-Metallic Materials
Phase behavior and Mechanical properties of polymer
Mechanical properties
The bulk
properties of a polymer are those most often of end-use interest. These are the
properties that dictate how the polymer actually behaves on a macroscopic
scale.
Tensile strength
The
tensile strength of a material quantifies how much stress the material will
endure before suffering permanent deformation. This is very important in
applications that rely upon a polymer's physical strength or durability. For
example, a rubber band with a higher tensile strength will hold a greater
weight before snapping. In general, tensile strength increases with polymer
chain length and crosslinking of polymer chains.
Young's modulus of elasticity
Young's
Modulus quantifies the elasticity of the polymer. It is defined, for small
strains, as the ratio of rate of change of stress to strain. Like tensile
strength, this is highly relevant in polymer applications involving the
physical properties of polymers, such as rubber bands. The modulus is strongly
dependent on temperature.
Transport
properties
Transport properties such as diffusivity relate to
how rapidly molecules move through the polymer matrix. These are very important
in many applications of polymers for films and membranes.
Phase behavior
Melting point
The term melting point, when applied to polymers,
suggests not a solid-liquid phase transition but a transition from a
crystalline or semi-crystalline phase to a solid amorphous phase. Though
abbreviated as simply Tm, the
property in question is more
properly
called the crystalline melting temperature. Among synthetic polymers,
crystalline melting is only discussed with regards to thermoplastics, as
thermosetting polymers will decompose at high temperatures rather than melt.
Glass transition temperature
A parameter of particular interest in synthetic
polymer manufacturing is the glass transition temperature (Tg), which describes
the temperature at which amorphous
polymers
undergo a transition from a rubbery, viscous amorphous solid, to a brittle,
glassy amorphous solid. The glass transition temperature may be engineered by
altering the degree of branching or crosslinking in the polymer or by the
addition of plasticizer.
Mixing behavior
In general, polymeric mixtures are far less
miscible than mixtures of small molecule materials. This effect results from
the fact that the driving force for mixing is usually entropy, not interaction
energy. In other words, miscible materials usually form a solution not because
their interaction with each other is more favorable than their
self-interaction, but because of an increase in entropy and hence free energy
associated with increasing the amount of volume available to each component.
This increase in entropy scales with the number of particles (or moles) being
mixed. Since polymeric molecules are much larger and hence generally have much
higher specific volumes than small molecules, the number of molecules involved
in a polymeric mixture is far smaller than the number in a small molecule
mixture of equal volume. The energetics of mixing, on the other hand, is
comparable on a per volume basis for polymeric and small molecule mixtures.
This tends to increase the free energy of mixing for polymer solutions and thus
make solvation less favorable. Thus, concentrated solutions of polymers are far
rarer than those of small molecules.
Furthermore, the phase behavior of polymer
solutions and mixtures is more complex than that of small molecule mixtures.
Whereas most small molecule solutions exhibit only an upper critical solution
temperature phase transition, at which phase separation occurs with cooling,
polymer mixtures commonly exhibit a lower critical solution temperature phase
transition, at which phase separation occurs with heating.
In dilute solution, the properties of the polymer
are characterized by the interaction between the solvent and the polymer. In a
good solvent, the polymer appears swollen and occupies a large volume. In this
scenario, intermolecular forces between the solvent and monomer subunits
dominate over intramolecular interactions. In a bad solvent or poor solvent,
intramolecular forces dominate and the chain contracts. In the theta solvent,
or the state of the polymer solution where the value of the second virial coefficient
becomes 0, the intermolecular polymer-solvent repulsion balances exactly the
intramolecular monomer-monomer attraction. Under the theta condition (also
called the Flory condition), the polymer behaves like an ideal random coil. The
transition between the states is known as a coil-globule transition.
Inclusion of
plasticizers
Inclusion of plasticizers tends to lower Tg and increase polymer
flexibility.
Plasticizers
are generally small molecules that are chemically similar to the polymer and
create gaps between polymer chains for greater mobility and reduced interchain
interactions. A good example of the action of plasticizers is related to
polyvinylchlorides or PVCs. A uPVC, or unplasticized polyvinylchloride, is used
for things such as pipes. A pipe has no plasticizers in it, because it needs to
remain strong and heat-resistant. Plasticized PVC is used for clothing for a
flexible quality. Plasticizers are also put in some types of cling film to make
the polymer more flexible.
Chemical properties
The attractive forces between polymer chains play a
large part in determining a polymer's properties. Because polymer chains are so
long, these interchain forces are amplified far beyond the attractions between
conventional molecules. Different side groups on the polymer can lend the
polymer to ionic bonding or hydrogen bonding between its own chains. These
stronger forces typically result in higher tensile strength and higher
crystalline melting points.
The intermolecular forces in polymers can be
affected by dipoles in the monomer units. Polymers containing amide or carbonyl
groups can form hydrogen bonds between adjacent chains; the partially
positively charged hydrogen atoms in N-H groups of one chain are strongly
attracted to the partially negatively charged oxygen atoms in C=O groups on
another. These strong hydrogen bonds, for example, result in the high tensile
strength and melting point of polymers containing urethane or urea linkages.
Polyesters have dipole-dipole bonding between the oxygen atoms in C=O groups
and the hydrogen atoms in H-C groups. Dipole bonding is not as strong as
hydrogen bonding, so a polyester's melting point and strength are lower than
Kevlar's (Twaron), but polyesters have greater flexibility.
Ethene,
however, has no permanent dipole. The attractive forces between polyethylene
chains arise from weak van der Waals forces. Molecules can be thought of as
being surrounded by a cloud of negative electrons. As two polymer chains
approach, their electron clouds repel one another. This has the effect of
lowering the electron density on one side of a polymer chain, creating a slight
positive dipole on this side. This charge is enough to attract the second
polymer chain. Van der Waals forces are quite weak, however, so polyethylene
can have a lower melting temperature compared to other polymers.
Standardized
polymer nomenclature
There are multiple conventions for naming polymer
substances. Many commonly used polymers, such as those found in consumer
products, are referred to by a common or trivial name. The trivial name is
assigned based on historical precedent or popular usage rather than a
standardized naming convention. Both the American Chemical Society (ACS) and
IUPAC have proposed standardized naming conventions; the ACS and UPAC
conventions are similar but not identical. Examples of the differences between
the various naming conventions are given in the table below:

In both
standardized conventions, the polymers' names are intended to reflect the
monomer(s) from which they are synthesized rather than the precise nature of
the repeating subunit. For example, the polymer synthesized from the simple
alkene ethene is called polyethylene, retaining the -ene suffix even though the double bond is removed during the
polymerization process:
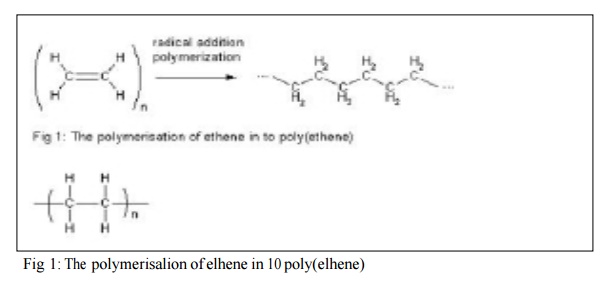
Fig 1:
The polymerisalion of elhene in 10 poly(elhene)
Polymer characterization
The characterization of a polymer requires several
parameters which need to be specified. This is because a polymer actually
consists of a statistical distribution of chains of varying lengths, and each
chain consists of monomer residues which affect its properties.
A variety
of lab techniques are used to determine the properties of polymers. Techniques
such as wide angle X-ray scattering, small angle X-ray scattering, and small
angle neutron scattering are used to determine the crystalline structure of
polymers. Gel permeation chromatography is used to determine the number average
molecular weight, weight average molecular weight, and polydispersity. FTIR,
Raman and NMR can be used to determine composition. Thermal properties such as
the glass transition temperature and melting point can be determined by
differential scanning calorimetry and dynamic mechanical analysis. Pyrolysis
followed by analysis of the fragments is one more technique for determining the
possible structure of the polymer. Thermogravimetry is a useful technique to
evaluate the thermal stability of the polymer. Detailed analyses of TG curves
also allow us to know a bit of the phase segregation in polymers. Rheological
properties are also commonly used to help determine molecular architecture
(molecular weight, molecular weight distribution and branching)as well as to
understand how the polymer will process, through measurements of the polymer in
the melt phase. Another polymer characterization technique is Automatic
Continuous Online Monitoring of Polymerization Reactions (ACOMP) which provides
real-time characterization of polymerization reactions. It can be used as an
analytical method in R&D, as a tool for reaction optimization at the bench
and pilot plant level and, eventually, for feedback control of full-scale
reactors. ACOMP measures in a model-independent fashion the evolution of
average molar mass and intrinsic viscosity, monomer conversion kinetics and, in
the case of copolymers, also the average composition drift and distribution. It
is applicable in the areas of free radical and controlled radical homo- and
copolymerization, polyelectrolyte synthesis, heterogeneous phase reactions,
including emulsion polymerization, adaptation to batch and continuous reactors,
and modifications of polymers.
Polymer degradation

A plastic
item with thirty years of exposure to heat and cold, brake fluid, and sunlight.
Notice the discoloration, swollen dimensions, and tiny splits running through
the material
Polymer
degradation is a change in the properties—tensile strength, color, shape, or
molecular weight—of a polymer or polymer-based product under the influence of
one or more environmental factors, such as heat, light, chemicals and, in some
cases, galvanic action. It is often due to the scission of polymer chain bonds
via hydrolysis, leading to a decrease in the molecular mass of the polymer.
Although
such changes are frequently undesirable, in some cases, such as biodegradation
and recycling, they may be intended to prevent environmental pollution.
Degradation can also be useful in biomedical settings. For example, a copolymer
of polylactic acid and polyglycolic acid is employed in hydrolysable stitches
that slowly degrade after they are applied to a wound.
The
susceptibility of a polymer to degradation depends on its structure. Epoxies
and chains containing aromatic functionalities are especially susceptible to UV
degradation while polyesters are susceptible to degradation by hydrolysis,
while polymers containing an unsaturated backbone are especially susceptible to
ozone cracking. Carbon based polymers are more susceptible to thermal
degradation than inorganic polymers such as polydimethylsiloxane and are
therefore not ideal for most high-temperature applications.
High-temperature
matrices S uch as bismaleimides (BMI), condensation polyimides (with an O-C-N
bond), triazines (with a nitrogen (N) containing ring), and blends thereof are
susceptible to polymer degradation in the form of galvanic corrosion when bare
carbon fiber reinforced polymer CFRP is in contact with an active metal such as
aluminum in salt water environments.
The
degradation of polymers to form smaller molecules may proceed by random
scission or specific scission. The degradation of polyethylene occurs by random
scission —a random breakage of the bonds that hold the atoms of the polymer
together. When heated above 450 °C, polyethylene degrades to form a mixture of
hydrocarbons. Other polymers, such as poly(alpha-methylstyrene), undergo
specific chain scission with breakage occurring only at the ends. They
literally unzip or depolymerize back to the constituent monomer.
The
sorting of polymer waste for recycling purposes may be facilitated by the use
of the Resin identification codes developed by the Society of the Plastics
Industry to identify the type of plastic.
Product failure

In a finished product, such a change is to be
prevented or delayed. Failure of safety- critical polymer components can cause
serious accidents, such as fire in the case of cracked and degraded polymer
fuel lines. Chlorine-induced cracking of acetal resin plumbing joints and
polybutylene pipes has caused many serious floods in domesticproperties,
especially in the USA in the 1990s. Traces of chlorine in the water supply
attacked vulnerable polymers in the plastic plumbing, a problem which occurs
faster if any of the parts have been poorly extruded or injection molded.
Attack of the acetal joint occurred because of faulty molding, leading to
cracking along the threads of the fitting which is a serious stress
concentration.
Polymer oxidation has caused accidents involving
medical devices. One of the oldest known failure modes is ozone cracking caused
by chain scission when ozone gas attacks susceptible elastomers, such as
natural rubber and nitrile rubber. They possess double bonds in their repeat
units which are cleaved during ozonolysis. Cracks in fuel lines can penetrate
the bore of the tube and cause fuel leakage. If cracking occurs in the engine
compartment, electric sparks can ignite the gasoline and can cause a serious
fire.
Fuel
lines can also be attacked by another form of degradation: hydrolysis. Nylon
6,6 is susceptible to acid hydrolysis, and in one accident, a fractured fuel
line led to a spillage of diesel into the road. If diesel fuel leaks onto the
road, accidents to following cars can be caused by the slippery nature of the
deposit, which is like black ice
Related Topics