Chapter: Mechanical : Engineering materials and metallurgy : Non-Metallic Materials
Polymer Architecture
POLYMER ARCHITECTURE
Branch point in a polymer
An important microstructural feature determining
polymer properties is the polymer architecture.The simplest polymer
architecture is a linear chain: a
single backbone with no branches. A related unbranching architecture is a ring polymer. A branched polymer
molecule is composed of a main chain with one or more substituent side chains
or branches. Special types of branched polymers include star polymers, comb
polymers, brush polymers, dendronized polymers, ladders, and dendrimers.
Branching of polymer chains affects the ability of
chains to slide past one another by altering intermolecular forces, in turn
affecting bulk physical polymer properties. Long chain branches may increase
polymer strength, toughness, and the glass transition temperature (Tg) due to
an increase in the number of entanglements per chain. The effect
of such
long-chain branches on the size of the polymer in solution is characterized by
the branching index. Random length and atactic short chains, on the other hand,
may reduce polymer strength due to disruption of organization and may likewise
reduce the crystallinity of the polymer.
A good
example of this effect is related to the range of physical attributes of
polyethylene. High-density polyethylene (HDPE) has a very low degree of
branching, is quite stiff, and is used in applications such as milk jugs. Low
-density polyethylene (LDPE), on the other hand, has significant numbers of
both long and short branc hes, is quite flexible, and is used in applications
such as plastic films.
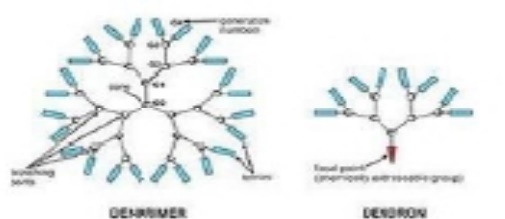
Dendrimers
are a special case of polymer where every monomer unit is branched. This tends
to reduce intermolecular chain entanglement and crystallization. Alternativel
y, dendritic polymers are not perfectly branched but share similar properties
to dendrimers due to their high degree of branching.
The architecture of the polymer is often physically
determined by the functionality of
the monomers from which it is formed. This property of a monomer is defined as
the number of reaction sites at which may form chemical covalent bonds. The
basic functionality required for forming even a linear chain is two bonding
sites. Higher functionality yields branched or even crosslinked or networked
polymer chains.
An effect related to branching is chemical
crosslinking - the formation of covalent bonds between chains. Crosslinking
tends to increase Tg and increase strength and
toughness.
Among other applications, this process is used to strengthen rubbers in a
process known as vulcanization, which is based on crosslinking by sulfur. Car
tires, for ex ample, are highly crosslinked in order to reduce the leaking of
air out of the tire and to toughen their durability. Eraser rubber, on the other
hand, is not crosslinked to allow flaking of the rubber and prevent damage to
the paper.
A cross-link suggests a branch point from which
four or more distinct chains emanate. A polymer molecule with a high degree of
crosslinking is referred to as a polymer network. Sufficiently high crosslink
concentrations may lead to the formation of an infinite network, also known as
a gel, in which networks of chains are of unlimited extent—essentially all
chains have linked into one molecule.
Chain length
The physical properties of a polymer are strongly
dependent on the size or length of the polymer chain. For example, as chain
length is increased, melting and boiling temperatures increase quickly. Impact
resistance also tends to increase with chain length, as does the viscosity, or
resistance to flow, of the polymer in its melt state.
Chain length is related to melt viscosity roughly
as 1:103.2, so that a tenfold increase in polymer chain length
results in a viscosity increase of over 1000 times. Increasing chain length
furthermore tends to decrease chain mobility, increase strength and toughness,
and increase the glass transition temperature (Tg).This is a result of the
increase
in chain interactions such as Van der Waals attractions and entanglements that
come with increased chain length. These interactions tend to fix the individual
chains more strongly in position and resist deformations and matrix breakup,
both at higher stresses and higher temperatures.
A common
means of expressing the length of a chain is the degree of polymerization,
which quantifies the number of monomers incorporated into the chain . As with
other molecules, a polymer's size may also be expressed in terms of molecular
weight. Since synthetic polymerization techniques typically yield a polymer
product including a range of molecular weights, the weight is often expressed
statistically to describe the distribution of chain lengths present in the
same. Common examples are the number average molecular weight and weight
average molecular weight. The ratio of these two values is the polydispersity
index, commonly used to express the "width" of the molecular weight
distribution. A final measurement is contour length, which can be understood as
the length of the chain backbone in its fully extended state.
The flexibility of an unbranched chain polymer is
characterized by its persistence length.
Monomer arrangement in copolymers

Monomers
within a copolymer may be organized along the backbone in a variety of ways.
•Alternating copolymers possess
regularly alternating monomer residues: [AB...]n (2). II Periodic copolymers
have monomer residue types arranged in a repeating sequence: [AnBm...] m being
different from n .
Statistical
copolymers have monomer residues arranged according to a know statistical rule.
A statistical copolymer in which the probability of finding a particular type
of monomer residue at an particular point in the chain is independent of the
types of surrounding monomer residue may be referred to as a truly random
copolymer (3). •Block copolymers have two or more homopolymer subunits linked
by covalent bonds (4). Polymers with two or three blocks of two distinct
chemical species (e.g., A and B) are called diblock copolymers and triblock
copolymers, respectively. Polymers with
three blocks, each of a different chemical species (e.g., A, B, and C) are
termed triblock terpolymers. Graft or grafted copolymers contain side chains
that have a different composition or configuration than the main chain.(5)
Tacticity
Tacticity
describes the relative stereochemistry of chiral centers in neighboring
structural units within a macromolecule. There are three types: isotactic (all
substituents on the same side), atactic (random placement of substituents), and
syndiotactic (alternating placement of substituents).
Related Topics