Chapter: Mechanical : Engineering materials and metallurgy : Non-Metallic Materials
Fundamentals of Ceramics
FUNDAMENTALS OF CERAMICS
Ionicandcovalent bonding
Ceramics
Ceramics (ceramic materials) are
non-metallic inorganic compounds formed from metallic (Al, Mg, Na, Ti, W) or
semi-metallic (Si, B) and non- metallic (O, N, C) elements.
Atoms of the elements are held together in a
ceramic structure by one of the following bonding mechanism: Ionic Bonding,
Covalent Bonding, Mixed Bonding (Ionic-Covalent).
Most of ceramic materials have a mixed bonding
structure with various ratios between Ionic and Covalent components. This ratio
is dependent on the difference in the electronegativities of the elements and
determines which of the bonding mechanisms is dominating ionic or covalent.
Electro negativity
Ionic Bonding
Covalent Bonding
Ionic-Covalent
(mixed) Bonding
Characterization of
ceramics properties
Electro negativity
Electro negativity is an
ability of atoms of the element to attract electrons of atoms of another element. Electronegativity is measured
in a relative dimensionless unit (Pauling scale) varying in a range between 0.7
(francium) to
3.98
(fluorine).
Non-metallic
elements are strongly electronegative. Metallic elements are characterized by
low electro negativity or high electro
positivity - ability of the element to lose electrons.
Ionic Bonding
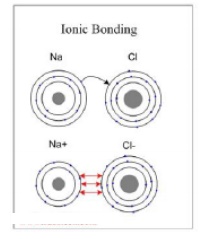
Ionic bonding occurs between two elements with a large difference
in their electro negativities (metallic and non-metallic), which become ions
(negative and positive) as a result of transfer of the valence electron from
the element with low electro negativity to the element with high electro
negativity.
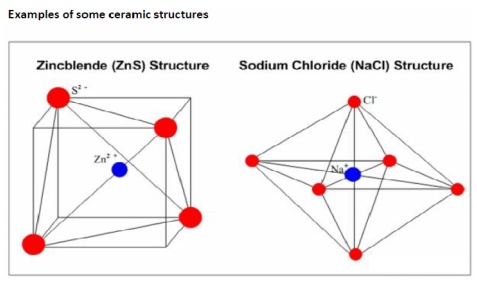
The typical example of a material with Ionic Bonding is sodium
chloride (NaCl).
Electropositive sodium atom donates its valence electron to the
electronegative chlorine atom, completing its outer electron level (eight
electrons):
As a result of the electron transfer the sodium atom becomes a
positively charged ion (cation) and the chlorine atom becomes a negatively
charged ion (anion). The two ions attract to each other by Coulomb force,
forming a compound (sodium chloride) with ionic bonding. Ionic bonding is
non-directional.
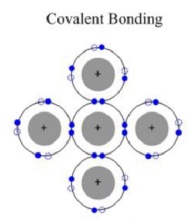
Covalent Bonding
Covalent bonding occurs between two elements with
low difference in their electronegativities
(usually non-metallics), outer electrons of which are shared between the four
neighboring atoms. Covalent Bonding is strongly directional.
Ionic-Covalent
(mixed) Bonding
Ionic-covalent
(mixed) bonding with various ratios of the two fractions (ionic and covalent) occurs in most of
ceramic materials.
Degree of
Ionic Bonding can be estimated from the following formula:
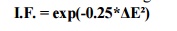
Where
I.F. -
fraction of ionic bonding;
ΔE -
difference in the electro negativities of the elements.
Characterization of ceramics
properties
In contrast to metallic bonding neither ionic nor covalent bonding
form free electrons, therefore ceramic materials have very low electric
conductivity and thermal conductivity. Since both ionic and covalent bonds are
stronger than metallic bond, ceramic materials are stronger and harder than
metals.
Strength
of ionic and covalent bonds also determines high melting point, modulus of elasticity (rigidity), temperature and chemical stability of ceramic
materials. Motion of dislocations through a ceramic structure is impeded
therefore ceramics are generally brittle
that limits their use as structural materials.
Ceramics
may have either crystalline or amorphous structure. There are also ceramic
materials, consisting of two constituents: crystalline and amorphous.
Structure of
ceramic materials
The following factors affect
structure of ceramics:
Balance of electrical charges of
anions and cations
Radius Ratio (rc/ra)
Where
rc - radius
of cation;
ra - radius
of anion.
Radius
Ratio determines Coordination Number
(CN)- the maximum number of anion nearest neighbors for a cation.The anion
neighbors do not touch each other.
rc/ra =
0.225…0.414(SiO2) CN = 4
rc/ra =
0.414…0.732(SnO2, PbO2) CN = 6
rc/ra =
0.732…1.0(ThO2) CN = 8
Covalent bonding component, which tends to form
tetrahedral coordination, may affect the Coordination Number.
•Ions structure are
packed with maximum
density.
Ceramic structures are classified and designated
according to the pattern structures of several natural minerals:
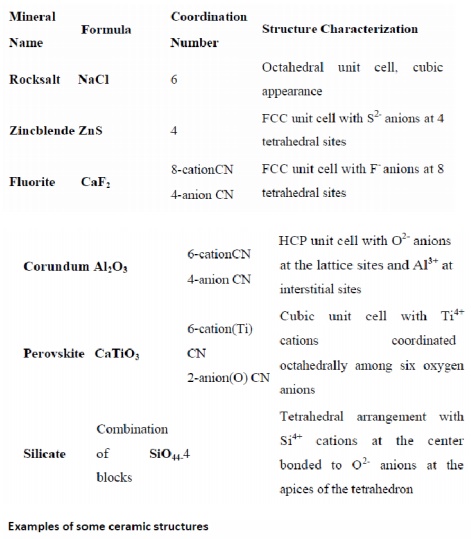
Tetrahedral silica block (SiO4-4) may form various
silicate structures:
Island and DoubleIsland Silicates
Single or two silica blocks, containing other
cations, form Island (olivine) or Double Island Silicates (hemimorphite).
Ring and Chain Structures
Several (3,4,5,6) silica units join each other,
forming a chain (orthopyroxenes, clinopyroxenes, asbestos) or closed ring
(beryl).
Sheet (layer) structure
Silica
units connect to each other, forming infinite sheet (micas, serpentine,
chlorite,
talc).
Framework silicate
Quartz, cristobalite, and tridymite minerals are
based on the framework silicate structure.
Silicates
exist in two forms: crystalline and amorphous (glasses).
General
classification ofceramics
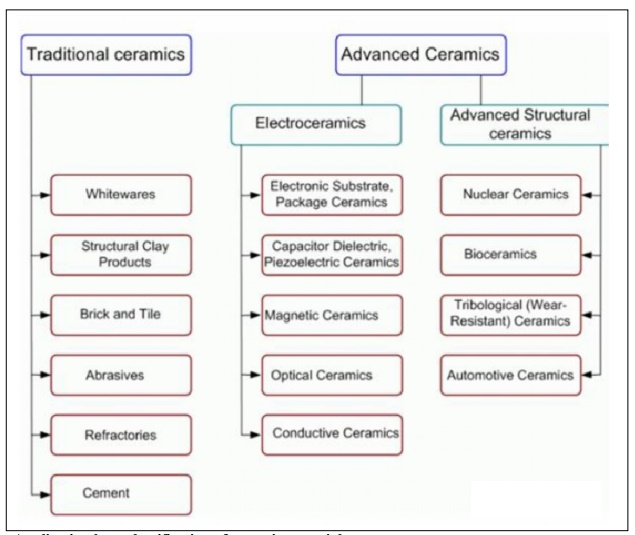
There are
various classification systems of ceramic materials, which may be attributed to
one of two principal categories: application
base system or composition base
system.
Application base classification
of ceramic materials
Tribology of ceramics
Characteristics
of friction and wear of a ceramic material are determined by a combination of
its bulk microstructure parameters, surface conditions and environmental
factors (temperature, atmosphere pressure, etc.), lubrication conditions.
Effect of
microstructure on tribological properties of ceramics
o
Parameters of microstructure and their influence on friction and wear of
ceramics o Manufacturing processes forming microstructure of ceramics
Effect of
surface characteristics on tribological properties of ceramics o Surface
characteristics
o Methods of modification of ceramic surfaces Effect of lubrication on tribological
properties of ceramics
Related Topics