Chapter: Mechanical : Engineering materials and metallurgy : Ferrous and Non Ferrous Metals
Ferrous and Non Ferrous Metals
FERROUS AND NON FERROUS METALS
1 Effect of alloying elements on steel properties
2 Characteristics of alloying elements
3 Maraging steels
4 Heat treatment cycle
5 Classificaion of copper and its alloys
5.1 Brasses
5.2 Bronze
5.3 Tool and die steel
6 Effects of alloying elements on steel
1.EFFECT OF ALLOYING ELEMENTS ON
STEEL PROPERTIES
Alloying
is changing chemical composition of steel by adding elements with purpose to
improve its properties as compared to the plane carbon steel.
The properties, which may be
improved
Stabilizing austenite -
increasing the temperature range, in which austenite exists.
The
elements, having the same crystal structure as that of austenite (cubic face
centered - FCC), raise the A4 point (the temperature of formation of austenite
from liquid phase) and decrease the A3 temperature. These elements are nickel
(Ni), manganese (Mn), cobalt (Co) and c opper (Cu).
Examples
of austenitic steels: austenitic stainless steels, Hadfield steel (1%C, 13%Mn,
1.2%Cr).
Stabilizing ferrite -
decreasing the temperature range, in which austenite exists.
The
elements, having the same crystal structure as that of ferrite (cubic body
centered - BCC), lower the A4 point and increase the A3 temperature.
These
elements lower the solubility of carbon in austenite, causing increase of
amount of carbides in the steel.
The
following elements have ferrite stabilizing effect: chromium (Cr),
tungsten
(W), Molybdenum (Mo), vanadium (V), aluminum (Al) and silicon (Si).
Examples
of ferritic steels:transformer sheets steel (3%Si), F-Cr alloy
Carbide forming -
elements forming hard carbides in steels.
The
elements like chromium (Cr), tungsten (W), molybdenum (Mo),vanadium (V),
titanium (Ti), niobium (Nb), tantalum (Ta),zirconium (Zr) form hard (often
complex) carbides, increasing steel hardness and strength.Examples of steels
containing relatively high concentration of carbides: hot work tool steels,
high speed steels. Carbide forming elements also form nitrides reacting with
Nitrogen in steels.
Graphitizing -
decreasing stability of carbides, promoting their breaking and formation of
free Graphite.
The
following elements have graphitizing effect: silicon (Si), nickel (Ni), cobalt
(Co), aluminum (Al).
Decrease of the eutectoid concentration.
The
following elements lower eutectoid concentration of carbon:
titanium
(Ti), molybdenum (Mo), tungsten (W), silicon (Si), chromium (Cr), nickel (Ni).
Increase of corrosion resistance.
Aluminum
(Al), silicon (Si), and chromium (Cr) form thin an strong oxide film on the
steel surface, protecting it from chemical attacks.
2.CHARACTERISTICS OF ALLOYING
ELEMENTS
Manganese
(Mn) - improves hardenability, ductility and wear resistance. Mn eliminates
formation of harmful iron sulfides, increasing strength at high temperatures.
Nickel
(Ni) - increases strength, impact strength and toughness, impart corrosion
resistance in combination with other elements.
Chromium
(Cr) - improves hardenability, strength and wear resistance, sharply increases
corrosion resistance at high concentrations (> 12%).
Tungsten
(W) - increases hardness particularly at elevated due to temperatures stable
carbides, refines grain size.
Vanadium
(V) - increases strength, hardness, creep resistance and impact resistance due
to formation of hard vanadium carbides, limits grain size.
Molybdenum
(Mo) - increases hardenability and strength particularly at high temperatures
and under dynamic conditions.
Silicon
(Si) - improves strength, elasticity, acid resistance and promotes large grain
sizes, which cause increasing magnetic permeability.
Titanium
(Ti) - improves strength and corrosion resistance, limits austenite grain size.
Cobalt
(Co) - improves strength at high temperatures and magnetic permeability.
Zirconium
(Zr) - increases strength and limits grain sizes.
Boron
(B) - highly effective hardenability agent, improves deformability and
machinability.
Copper
(Cu) - improves corrosion resistance.
Aluminum
(Al) - deoxidizer, limits austenite grains growth.
3.MARAGING STEELS
Maraging
steels (from martensitic and aging) are steels (iron alloys) which are known
for possessing superior strength and toughness without losing malleability,
although they cannot hold a good cutting edge. Aging refers to the extended
heat-treatment process. These steels are a special class of low- carbon ultra-
high-strength steels which derive their strength not from carbon, but from
precipitation of inter-metallic compounds. The principal alloying element is 15
to 25% nickel. Secondary alloying elements are added to produce intermetallic
precipitates, which include cobalt, molybdenum, and titanium. Original
development was carried out on 20 and 25% Ni steels to which small additions of
Al, Ti, and Nb were made.
The
common, non-stainless grades contain 17-19% nickel, 8-12% cobalt,3-5%
molybdenum, 0.2-1.6% titanium. Addition of chromium and produces stainless
grades resistant to corrosion. This also indirectly increases hardenability as
they require less nickel: high-chromium, high-nickel steels are generally
austenitic and unable to transform to martensite when heat treated, while
lower-nickel steels can transform to martensite.
Properties
Due
to the low carbon content maraging steels have good machinability. Prior t o aging,
they may also be cold rolled to as much as 80- 90% without cracking. Maraging
steels offer good weldability, but must be aged afterward to restore the
properties of heat affected zone. When heat-treated the alloy has very little
dimensional change, so it is often machined to its final dimensions. Due to the
high alloy content maraging steels have a high hardenability. Since ductile
FeNi martensites are formed upon cooling, cracks are non-existent or
negligible. The steels can be nitrided to increase case hardness, and polished
to a fine surface finish.
Non-stainless
varieties of maraging steel are moderately corrosion- resistant, and resist
stress corrosion and hydrogen embrittlement. Corrosion- resistance can be
increased by cadmium plating or phosphating.
4.HEAT TREATMENT CYCLE
The
steel is first annealed at approximately 820 °C (1,510 °F) for 15- 30 minutes
for thin sections and for 1 hour per 25 mm thickness for heavy sections, to
ensure formation of a fully austenitized structure. This is followed by air
cooling to room temperature to form a soft, heavily-dislocated iron-nickel lath
(untwinned) martensite. Subsequent aging (precipitation hardening) of the more
common alloys for approximately 3 hours at a temperature of 480 to 500 °C
produces a fine dispersion of Ni3(X,Y) intermetallic phases along dislocations
left by martensitic transformation, where X and Y are solute elements added for
such precipitation. Overaging leads to a reduction in stability of the primary,
metastable, coherent precipitates, leading to their dissolution and replacement
with semi-coherent Laves phases such as Fe2Ni/Fe2Mo. Further excessive heat-
treatment brings about the decomposition of the martensite and reversion to
austenite.
Newer
compositions of maraging steels have revealed other intermetallic
stoichiometries and crystallographic relationships with the parent martensite,
including rhombohedral and massive complex Ni50(X,Y,Z)50 (Ni50M50 in simplified
notation).
Uses
Maraging
steel's strength and malleability in the pre-aged stage allows it to be formed
into thinner rocket and missile skins than other steels, reducing weight for a
given strength. Maraging steels have very stable properties, and, even after
overaging due to excessive temperature, only soften slightly. These alloys
retain their properties at mildly elevated operating temperatures and have
maximum service temperatures of over 400 °C (752 °F)
They
are suitable for engine components, such as crankshafts and gears, and the
firing pins of automatic weapons that cycle from hot to cool repeatedly while
under substantial load. Their uniform expansion and easy machinability before
aging make maraging steel useful in high-wear
components
of assembly lines and dies. Other ultra-high-strength steels, such as Aermet
alloys, are not as machinable because of their carbide content.
In the sport of fencing, blades used in
competitions run under the auspices of the Fédération Internationale d'Escrime
are often made with maraging steel. Maraging blades are required in foil and
épée because crack propagation in maraging steel is 10 times slower than in
carbon steel, resulting in less blade breakage and fewer injuries. The notion
that such blades break flat is a fencing urban legend: testing has shown that
the blade-breakage patterns in carbon steel and maraging steel blades are
identical.
Stainless maraging steel is used in bicycle
frames and golf club heads. It is also used in surgical components and
hypodermic syringes, but is not suitable for scalpel blades because the lack of
carbon prevents it from holding a good cutting edge. Maraging steel production,
import, and export by certain states, such as the
United
States, is closely monitored by international authorities because it is
particularly suited for use in gas centrifuges for uranium enrichment; lack of
maraging steel significantly hampers this process. Older centrifuges used
aluminum tubes; modern ones, carbon fiber composite.
Copper alloys
are metal alloys that have copper as their principal component. They have high
resistance against corrosion. The best known traditional types are bronze,
where tin is a significant addition, and brass, using zinc instead. Both these
are imprecise terms, and today the term copper alloy tends to be substituted,
especially by museums.
Compositions
The similarity in external appearance of the
various alloys, along with the different combinations of elements used when
making each alloy, can lead to confusion when categorizing the different
compositions. There are as many as 400 different copper and copper-alloy
compositions loosely grouped into the categories: copper, high copper alloy,
brasses, bronzes, copper nickels, copper-nickel-zinc (nickel silver), leaded
copper, and special alloys. The following table lists the principal alloying
element for four of the more common types used in modern industry, along with
the name for each type. Historical types, such as those that characterize the
Bronze Age, are vaguer as the mixtures were generally variable.
5.CLASSIFICATION OF COPPER AND ITS
ALLOYS
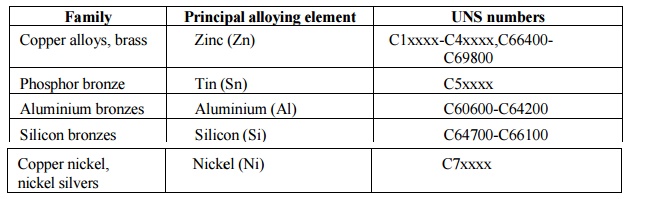
1.BRASSES
A brass is an alloy of copper with zinc.
Brasses are usu ally yellow in color. The zinc content can vary between few %
to about 40%; as long as it is kept under 15%, it does not markedly decrease
corrosion resistance of copper. Brasses can be se nsitive to selective leaching
corrosion under certain conditions, when zinc is leached from the alloy
(dezincification), leaving behind a spongy copper structure.
2.BRONZES
A bronze is an alloy of copper and other
metals, most often tin, but also aluminium and silicon.
Aluminium bronzes are alloys of copper and
aluminium. The content of aluminium ranges mostly between 5-11%. Iron, nickel,
manganes e and silicon are sometimes added. Th ey have higher strength and
corrosion resistance than other bronzes, especially in m arine environment, and
have low reactivity to sulfur compounds. Aluminium forms a thin passivation
layer on the surface of the metal.
CARBON STEELS
Carbon steels are iron-carbon alloys
containing up to 2.06% of carbon, up to 1.65% of manganese, up to 0.5% of
silicon and sulfur and phosphorus as impurities. Carbon content in carbon steel
determines its strength and ductility. The higher carbon content, the higher steel
strength and the lower its ductility.
ALLOY STEELS
Alloy steels are ir on-carbon alloys, to which
alloying elements are added with a purpose to improve the steels properties as
com pared to the Carbon steels. Due to effect of alloying elements, properties
of alloy steels exceed those of plane carbon steels. AISI/SAE classification
divide alloy steels
According
to the four-digit classification SAE/AISI system: First digit indicates the
class of the alloy steel:
2-
Nickel steels;
3-Nickel-chromiu
m steels;
4-
Molybdenum steels;
5-
Chromium steels;
6-Chromium-vanadium
steels;
7-Tungsten-chrom
ium steels;
9-
Silicon-manganese steels.
Second
digit indicates concentration of the major element in percents (1 means 1%).
Last
two digits indicate carbon concentration in 0,01%.
Example:
SAE 5130 means alloy chromium steel, containing 1% of chromium and 0.30% of
carbon.
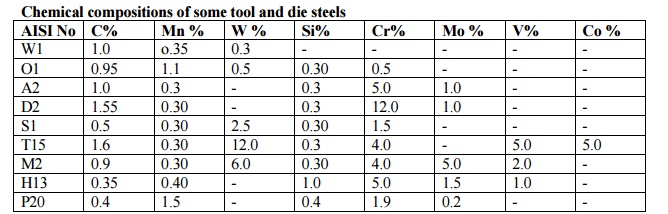
3.TOOL AND DIE STEELS
Tool
and die steels are high carbon steels (either carbon or alloy) possessing high
hardness, strength and wear resistance. Tool steels are heat treatable. In
order to increase hardness and wear resistance of tool steels, alloying
elements forming hard and stable carbides (chromium, tungsten, vanadium,
manganese, molybdenum) are added to the composition. Designation system of
one-letter in combination with a number is accepted for tool steels. The letter
means:W - Water hardened plain carbon tool steels
Applications:
chisels, forging dies, hummers, drills, cutters, shear blades, cutters, drills,
razors.
Properties:
low cost, very hard, brittle, relatively low harden ability, suitable for small
parts working at not elevated temperatures.
O,
A, D - Cold work tool steels
Applications:
drawing and forging dies, shear blades, highly effective cutters. Properties:
strong, hard and tough crack resistant.
O
-Oil hardening cold work alloy steels;
A
-Air hardening cold work alloy steels;
D
-Diffused hardening cold work alloy steels;
S
- Shock resistant low carbon tool steels
Applications:
tools experiencing hot or cold impact.
Properties:
combine high toughness with good wear resistance.
T,M
– High speed tool steels (T-tungsten, M-molybdenum)
Applications:
cutting tools. Properties: high wear heat and shock resistance.
H
– Hot work tool steels
Applications:
parts working at elevated temperatures, like extrusion, casting and forging
dies. Properties: strong and hard at elevated temperatures.
P
- Plastic mold tool steels
Applications:
molds for injection molding of plastics.
Properties:
good machinability.
Chemical compositions of some tool and die steels
6.EFFECTS OF ALLOYING ELEMENTS IN
STEEL
Steel
is basically iron alloyed to carbon with certain additional elements to give
the required properties to the finished melt. Listed below is a summary of the
effects various alloying elements in steel.
Carbon
The
basic metal, iron, is alloyed with carbon to make steel and has the effect of
increasing the hardness and strength by heat treatment but the addition of
carbon enables a wide range of hardness and strength.
Manganese
Manganese
is added to steel to improve hot working properties and increase strength,
toughness and hardenability. Manganese, like nickel, is an austenite forming
element and has been used as a substitute for nickel in the A.I.S.I 200 Series
of Austenitic stainless steels (e.g. A.I.S.I 202 as a substitute for A.I.S.I
304)
Chromium
Chromium is added to the steel to increase
resistance to oxidation. This resistance increases as more chromium is added. 'Stainless
Steel' has approximately 11% chromium and a very marked degree of general
corrosion resistance when compared with steels with a lower percentage of
chromium. When added to low alloy steels, chromium can increase the response to
heat treatment, thus improving hardenability and strength.
Nickel
Nickel
is added in large amounts, over about 8%, to high chromium stainless steel to
form the most important class of corrosion and heat resistant steels. These are
the austenitic stainless steels, typified by 18-8, where the tendency of nickel
to form austenite is responsible for a great toughness and high strength at
both high and low temperatures. Nickel also improves resistance to oxidation
and corrosion. It increases toughness at low temperatures when added in smaller
amounts to alloy steels.
Molybdenum
Molybdenum,
when added to chromium-nickel austenitic steels, improves resistance to pitting
corrosion especially by chlorides and sulphur chemicals. When added to low
alloy steels, molybdenum improves high temperature strengths and hardness. When
added to chromium steels it greatly diminishes the tendency of steels to decay
in service or in heat treatment.
Titanium
The
main use of titanium as an alloying element in steel is for carbide
stabilisation. It combines with carbon to for titanium carbides, which are
quite stable and hard to dissolve in steel, this tends to minimise the
occurrence of inter-granular corrosion, as with A.I.S.I 321, when adding
approximately 0.25%/0.60% titanium, the carbon combines with the titanium in
preference to chromium, preventing a tie -up of corrosion resisting chromium as
inter-granular carbides and the accompanying loss of corrosion resistance at
the grain boundaries.
Phosphorus
Phosphorus
is usually added with sulphur to improve machinability in low alloy steels,
phosphorus, in small amounts, aids strength and corrosion resistance.
Experimental work shows that phosphorus present in austenitic stainless steels
increases strength. Phosphorus additions are known to increase the tendency to
cracking during welding.
Sulphur
When
added in small amounts sulphur improves machinability but does not cause hot
shortness. Hot shortness is reduced by the addition of manganese, which
combines with the sulphur to form manganese sulphide. As manganese sulphide has
a higher melting point than iron sulphide, which would form if manganese were
not present, the weak spots at the grain boundaries are greatly reduced during
hot working.
Selenium
Selenium
is added to improve machinability.
Niobium (Columbium)
Niobium
is added to steel in order to stabilise carbon, and as such performs in the
same way as described for titanium. Niobium also has the effect of
strengthening steels and alloys for high temperature service.
Nitrogen
Nitrogen
has the effect of increasing the austenitic stability of stainless steels and
is, as in the case of nickel, an austenite forming element. Yield strength is
greatly improved when nitrogen is added to austenitic stainless steels.
Silicon
Silicon
is used as a deoxidising (killing) agent in the melting of steel, as a result,
most steels contain a small percentage of silicon. Silicon contributes to
hardening of the ferritic phase in steels and for this reason silicon killed
steels are somewhat harder and stiffer than aluminium killed steels.
Cobalt
Cobalt
becomes highly radioactive when exposed to the intense radiation of nuclear
reactors, and as a result, any stainless steel that is in nuclear service will
have a cobalt restriction, usually aproximately 0.2% maximum. This problem is
emphasised because there is residual cobalt content in the nickel used in
producing these steels.
Tantalum
Chemically
similar to niobium and has similar effects.
Copper
Copper
is normally present in stainless steels as a residual element. However it is
added to a few alloys to produce precipitation hardening properties.
Related Topics