Chapter: Medical Surgical Nursing: Pain Management
Pharmacologic Interventions - Pain Management Strategies
Pain Management Strategies
Reducing pain to a “tolerable” level was once considered the goal of
pain management. However, even patients who have described pain relief as
adequate often report disturbed sleep and marked distress because of pain. In
view of the harmful effects of pain and inadequate pain management, the goal of
tolerable pain has been replaced by the goal of relieving the pain. Pain
management strategies include both pharmacologic and nonpharmacologic
ap-proaches. These approaches are selected on the basis of the pa-tient’s
requirements and goals. Appropriate analgesic medications are used as prescribed.
They are not considered a last resort to be used only when other pain relief
measures fail. Any intervention is most successful if initiated before pain
sensitization occurs, and the greatest success is usually achieved if several
interventions are applied simultaneously.
PHARMACOLOGIC
INTERVENTIONS
Managing a patient’s pain pharmacologically is accomplished in
collaboration with the physician or other primary care provider, the patient,
and often the family. The physician or nurse practi-tioner prescribes specific
medications for pain or may insert an intravenous line for administering
analgesic medications. Alter-natively, an anesthesiologist or nurse anesthetist
may insert an epidural catheter for their administration. However, it is the
nurse who maintains the analgesia, assesses its effectiveness, and reports if
the intervention is ineffective or produces side effects.
The pharmacologic management of pain requires close col-laboration and
effective communication among health care pro-viders. In the home setting, it
is often the family who manages the patient’s pain and assesses the
effectiveness of pharmacologic in-terventions, while it is the home care nurse
who evaluates the ad-equacy of pain relief strategies and the family’s ability
to manage the pain. The home care nurse reinforces teaching and ensures
communication among the patient, family care providers, physi-cian, pharmacist,
and other health care providers involved in the patient’s care.
Premedication Assessment
Before administering any
medication, the nurse asks the patient about allergies to medications and the
nature of any previous al-lergic responses. True allergic or anaphylactic
responses to opi-oids are rare, but it is not uncommon for a patient to report
an allergy to one of the opioids. On further examination, the nurse often
learns that the extent of the allergy was “itching” or “nau-sea and vomiting.”
These responses are not allergies; rather, they are side effects that, when
necessary, can be managed while the patient’s pain is relieved. The patient’s
description of responses or reactions should be documented and reported before
admin-istering the medication.
The nurse obtains the patient’s medication history (eg, cur-rent, usual,
or recent use of prescription or over-the-counter med-ications or herbal
agents), along with a history of health problems. Certain medications or
conditions may affect the analgesic med-ication’s effectiveness or the
metabolism and excretion of anal-gesic agents. Before administering analgesic
agents, the nurse should assess the patient’s pain status, including the
intensity of current pain, changes in pain intensity after the previous dose of
medication, and side effects of the medication.
Approaches for Using Analgesic Agents
Medications are most effective
when the dose and interval be-tween doses are individualized to meet the
patient’s needs. The only safe and effective way to administer analgesic
medications is by asking the patient to rate the pain and by observing the
re-sponse to medications.
BALANCED ANESTHESIA
Pharmacologic
interventions are most effective when a multi-modal or balanced analgesia
approach is used. Balanced analge-sia refers
to use of more than one form of analgesia concurrentlyto obtain more pain
relief with fewer side effects. Three general categories of analgesic agents
are opioids, NSAIDs, and local anesthetics. These agents work by different
mechanisms. Using two or three types of agents simultaneously can maximize pain
re-lief while minimizing the potentially toxic effects of any one agent. When
one agent is used alone, it usually must be used in a higher dose to be
effective. In other words, although it might re-quire 15 mg morphine to relieve
a certain pain, it may take only 8 mg morphine plus 30 mg ketorolac (an NSAID)
to relieve the same pain.
PRO RE NATA (PRN)
In the past, the
standard method used by most nurses and physi-cians in administering analgesia
was to administer the analgesic pro re
nata (PRN), or “as needed.” The standard practice was forthe nurse to wait
for the patient to complain of pain and then ad-minister analgesia. As a
result, many patients remained in pain because they did not know they needed to
ask for medication or waited until the pain became intolerable.
By its very nature, the
PRN approach to analgesia leaves the pa-tient sedated or in severe pain much of
the time. To receive pain relief from an opioid analgesic, the serum level of
that opioid must be maintained at a minimum therapeutic level (Fig. 13-8). By
the time the patient complains of pain, the serum opioid level is below the
therapeutic level. From the time the patient requests pain medication until the
nurse administers the medication, the patient’s serum level continues to fall.
The lower the serum opi-oid level, the more difficult it is to achieve the
therapeutic level with the next dose. The only way to ensure significant
periods of analgesia, using this method, is to give doses large enough to
pro-duce periods of sedation.
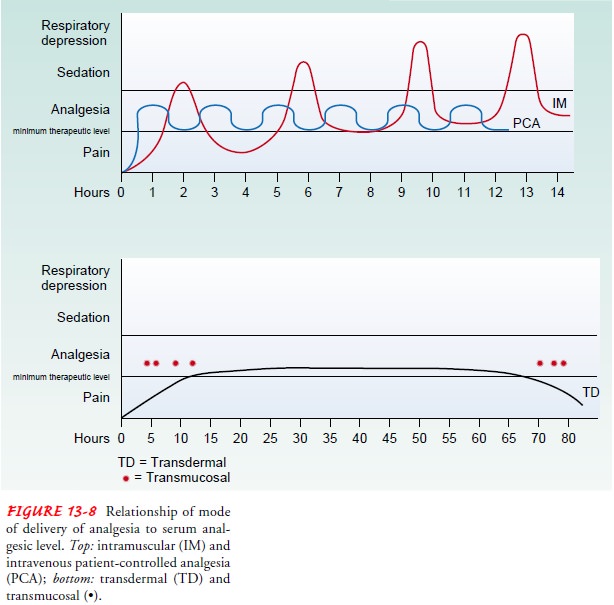
PREVENTIVE APPROACH
Currently, a preventive
approach to relieving pain by adminis-tering analgesic agents is considered the
most effective strategy because a therapeutic serum level of medication is
maintained. With the preventive approach, analgesic agents are administered at
set intervals so that the medication acts before the pain be-comes severe and
before the serum opioid level falls to a sub-therapeutic level.
Administering analgesic medication on a time basis, rather than on the basis of the patient’s report of pain, prevents the serum drug level from falling to subtherapeutic levels. An exam-ple of this would be giving the patient the prescribed morphine or the prescribed NSAID (ibuprofen) every 4 hours rather than waiting until the patient complains of pain.
If the patient’s pain is likely to occur around the clock or for a great portion of a 24-hour period, a regular around-the-clock schedule of administering analgesia may be indicated. Even if the analgesic is prescribed PRN, it can be administered on a preventive basis before the patient is in severe pain,
as long as the prescribed interval between doses is observed. The preventive
approach reduces the peaks and troughs in the serum level and provides more
pain relief for the patient with fewer adverse effects.
Smaller doses of medication are needed with the preventive approach
because the pain does not escalate to a level of severe in-tensity. Thus, a
preventive approach may result in the adminis-tration of less medication over a
24-hour period, thereby helping prevent tolerance to analgesic agents and
decreasing the severity of side effects (eg, sedation and constipation). Better
pain control can be achieved with a preventive approach, reducing the amount of
time the patient spends in pain.
In using the preventive approach, the nurse assesses the patient for
sedation before administering the next dose. The goal is to provide analgesia
before the pain becomes severe. It would not be safe to medicate a patient
(with an opioid) repeatedly if he or she was sedated or having no pain. It may
be necessary to decrease the dosage of the opioid analgesic so that the patient
receives pain relief with less sedation.
INDIVIDUALIZED DOSAGE
The dosage and the
interval between doses should be based on the patient’s requirements rather
than on an inflexible standard or routine. People metabolize and absorb
medications at differ-ent rates and experience different levels of pain.
Therefore, one dose of an opioid medication given at specified intervals may be
effective for one patient but ineffective for another.
Because of the fear of promoting addiction or causing respira-tory
depression, health care providers tend to prescribe and ad-minister inadequate
dosages of opioid agents to treat acute pain or chronic pain in the terminally
ill patient (Chart 13-5). How-ever, even prolonged administration of opioid
agents is associatedwith an extremely low incidence (less than 1%) of
addiction. Fur-thermore, small doses are not necessarily safe doses. For
example, some patients receiving a relatively small dose (25 to 50 mg) of
meperidine (Demerol) intramuscularly have experienced respira-tory depression,
whereas other patients have not exhibited any se-dation or respiratory
depression with very large doses of opioids.

Therefore, the effects of opioid analgesic medications must be
monitored, especially when the first dose is given or when the dose is changed
or given more frequently. The time, date, the pa-tient’s pain rating (scale of
0 to 10), the analgesic agent, other pain relief measures, side effects, and
patient activity are recorded. When the first dose of an analgesic is
administered, the nurse needs to record a pain rating score, blood pressure,
and respira-tory and pulse rates (all of which are considered “vital signs”).
If the pain has not decreased in 30 minutes (sooner if an intra-venous route is
used) and the patient is reasonably alert and has a satisfactory respiratory
status, blood pressure, and pulse rate, then some change in analgesia is
indicated. Although the dose of analgesic medication is safe for this patient,
it is ineffective in re-lieving the pain. Therefore, another dose of medication
may be indicated. In such instances, the nurse consults with the physi-cian to
determine what further action is warranted.
PATIENT-CONTROLLED ANALGESIA
Used to manage
postoperative pain as well as chronic pain, patient-controlled analgesia (PCA) allows patients to control the
ad-ministration of their own medication within predetermined safety limits.
This approach can be used with oral analgesic agents as well as with continuous
infusions of opioid analgesic agents by intra-venous, subcutaneous, or epidural
routes. PCA can be used in the hospital or home setting.
The PCA pump permits the patient to self-administer contin-uous infusions
of medication (basal rates) safely and to administer extra medication (bolus
doses) with episodes of increased pain or painful activities. A PCA pump is
electronically controlled by a timing device. Patients experiencing pain can
administer small amounts of medication directly into their intravenous,
sub-cutaneous, or epidural catheter by pressing a button. The pump then
delivers a preset amount of medication.
The PCA pump also can be
programmed to deliver a constant, background infusion of medication or basal
rate and still allow the patient to administer additional bolus doses as
needed. The timer can be programmed to prevent additional doses from being
administered until a specified time period has elapsed (lock-out time) and
until the first dose has had time to exert its maximal ef-fect. Even if the
patient pushes the button multiple times in rapid succession, no additional
doses are released. If another dose is re-quired at the end of the delay
period, the button must be pushed again to receive the dose. Patients who are
controlling their own opioid administration usually become sedated and stop
pushing the button before any significant respiratory depression occurs.
Nevertheless, assessing respiratory status remains a major role for the nurse.
A continuous infusion
plus bolus doses may be effective with cancer patients who require large doses
of analgesia, or for post-surgical patients. Although this allows more
uninterrupted sleep, the risk of sedation increases, especially when the
patient has min-imal or decreasing pain.
Patients who use PCA
achieve better pain relief (Walder, Schafer, Henzi et al., 2001) and often
require less pain medica-tion than those who are treated in the standard PRN
fashion. Because the patient can maintain a near-constant level of med-ication,
the periods of severe pain and sedation that occur with the traditional PRN
regimen are avoided.
To initiate PCA or any analgesia used at home or in the hos-pital, it is
important to avoid playing “catch-up.” Pain should be brought under control
before PCA starts, often by the use of an initial, larger bolus dose or loading
dose. Then, after control is achieved, the pump is programmed to deliver small
doses of medication at a time. If the patient with severe pain has a low serum level
of opioid analgesic because of an inadequate basal rate, it is difficult to
regain control with the small doses available by pump. Before the PCA pump is
used, repeated bolus doses of an intra-venous opioid may be administered as
prescribed over a short time until the pain is relieved. Then PCA is initiated.
If pain con-trol is not achieved with the maximal dose of medication
pre-scribed, further prescriptions are obtained. The goal is to achieve a
minimum therapeutic level of analgesia and to allow the patient to maintain
that level by using the PCA pump. The patient is in-structed not to wait until
the pain is severe before pushing the button to obtain a bolus dose. The
patient is also reminded not to become so distracted by an activity or visitor
that he or she for-gets to self-administer a prescribed dose of medication. One
po-tential drawback to distraction is that a patient who is using a PCA pump
may not self-administer any analgesia during the time of effective distraction.
When distraction ends suddenly (eg, the movie ends or the visitors leave), the
patient may be left without a therapeutic serum opioid level. When intermittent
distraction is used for pain relief, a continuous low-level background infusion
of opioid through the PCA pump may be prescribed so that when the distraction
ends, it will not be necessary to try to catch up.
If PCA is to be used in
the patient’s home, the patient and family are taught about the operation of
the pump and the side effects of the medication and strategies to manage them.
Local Anesthetic Agents
Local anesthetics work by blocking nerve conduction when ap-plied
directly to the nerve fibers. They can be applied directly to the site of
injury (eg, a topical anesthetic spray for sunburn) or di-rectly to nerve fibers
by injection or at the time of surgery. They can also be administered through
an epidural catheter.
TOPICAL APPLICATION
Local anesthetic agents have been successful in reducing the pain
associated with thoracic or upper abdominal surgery when in-jected by the
surgeon intercostally. Local anesthetic agents are rapidly absorbed into the
bloodstream, resulting in decreased availability at the surgical or injury site
and an increased anes-thetic level in the blood, increasing the risk of
toxicity. Therefore, a vasoconstrictive agent (eg, epinephrine or
phenylephrine) is added to the anesthetic agent to decrease its systemic
absorption and to maintain its concentration at the surgical or injury site.
A topical anesthetic
agent known as eutectic mixture or emul-sion of local anesthetics, or EMLA
cream, has been effective in preventing the pain associated with invasive
procedures such as lumbar puncture or the insertion of intravenous lines. To be
ef-fective, EMLA must be applied to the site 60 to 90 minutes be-fore the
procedure.
INTRASPINAL ADMINISTRATION
Intermittent or continuous administration of local anesthetic agents
through an epidural catheter has been used for years to produce anesthesia
during surgery. Although the administration of local anesthetic agents in the
spinal canal is still largely con-fined to acute pain, such as postoperative
pain and pain associ-ated with labor and delivery, the epidural administration
of local anesthetic agents for pain management is increasing.
A local anesthetic agent administered through an epidural catheter is
applied directly to the nerve root. The anesthetic agent can be administered
continuously in low doses, intermittently on a schedule, or on demand as the
patient requires it, and is often combined with the epidural administration of
opioids. Surgical patients treated with this combination experience fewer
compli-cations after surgery, ambulate sooner, and have shorter hospital stays
than patients receiving standard therapy (Correll, Viscusi, Grunwald et al., 2001).
Opioid Analgesic Agents
Opioids can be administered by various routes, including oral,
in-travenous, subcutaneous, intraspinal, intranasal, rectal, and trans-dermal
routes. The goal of administering opioids is to relieve pain and improve
quality of life; therefore, the route of administration, dose, and frequency of
administration are determined on an in-dividual basis. Factors that are
considered in determining the route, dose, and frequency of medication include
the character-istics of the pain (eg, its expected duration and severity), the
overall status of the patient, the patient’s response to analgesic medications,
and the patient’s report of pain. Although the oral route is usually preferred
for administering opioids, oral opioids must be given frequently enough and in
large enough doses to be effective. Opioid analgesic agents given orally may
provide a more consistent serum level than those given intramuscularly.
If the patient is
expected to require opioid analgesic agents at home, the patient’s and the
family’s ability to administer opioids as prescribed is considered in planning.
Steps are taken to ensure that the medication will be available to the patient.
Many phar-macies, especially those in smaller rural areas or inner cities, may
be reluctant to stock large amounts of opioids. Therefore, arrange-ments for
obtaining these prescription medications must be made ahead of time.
With the administration
of opioids by any route, side effects must be considered and anticipated.
Anticipating side effects and taking steps to minimize them increase the
likelihood that the pa-tient will receive adequate pain relief without
interrupting ther-apy to treat these effects.
RESPIRATORY DEPRESSION AND SEDATION
Respiratory depression is the most serious adverse effect of opi-oid
analgesic agents administered by intravenous, subcutaneous, or epidural routes.
However, it is relatively rare because doses ad-ministered through these routes
are small, and tolerance to respi-ratory depressant effects increases if the
dose is increased slowly. The risk of respiratory depression increases with age
and the con-comitant use of other opioids or other central nervous system
de-pressants. The risk of respiratory depression also increases when the
catheter is placed in the thoracic area and when the intra-abdominal or
intrathoracic pressure is increased.
The patient receiving opioids by any route must be assessed fre-quently
for changes in respiratory status. Specific notable changes are decreasing
respiratory rate or shallow respirations. Despite the risks associated with
their use, intravenous and epidural opioids are considered safe, with the risks
related to epidural administra-tion no greater than those related to
intravenous or other systemic routes of administration. Sedation, which may
occur with any method of administering opioids, is likely to occur when opioid
doses are increased. However, the patient often develops tolerance quickly, so
that in a short time the patient is no longer sedated by the dose that initially
caused sedation. Increasing the time between doses or reducing the dose
temporarily, as prescribed, usually prevents deep sedation from occurring. The
patient at risk for se-dation must be monitored closely for changes in
respiratory sta-tus. The patient is also at risk for other problems associated
with sedation and immobility. Therefore, the nurse must initiate strategies to
prevent problems such as skin breakdown.
NAUSEA AND VOMITING
Nausea and vomiting frequently occur with opioid use. Usually these
effects occur some hours after the initial injection. Patients, especially
postoperative patients, may not think to tell the nurse that they are
nauseated, particularly if the nausea is mild. How-ever, the patient receiving
an opioid should be assessed for nau-sea and vomiting, which may be triggered
by a position change and may be prevented by having the patient change
positions slowly. Adequate hydration and the administration of antiemetic
agents may decrease the incidence. Opioid-induced nausea and vomiting often
subside within a few days.
CONSTIPATION
Constipation, a common side effect of opioid use, may become so severe
that the patient is forced to choose between relief of pain and relief of
constipation. This situation can occur in patients after surgery and in
patients receiving large doses of opioids to treat cancer-related pain.
Preventing constipation must be a high priority in all patients receiving
opioids. Whenever a patient re-ceives opioids, a bowel regimen should begin at
the same time. Tolerance to this side effect does not occur; rather, it
persists even with long-term use of opioids.
Several strategies may help prevent and treat opioid-related
constipation. Mild laxatives and a high intake of fluid and fiber may be
effective in managing mild constipation. Unless con-traindicated, a mild
laxative and a stool softener should be ad-ministered on a regular schedule.
Continued severe constipation, however, often requires the use of a stimulating
cathartic agent, such as senna derivatives (Senokot) or bisacodyl (Dulcolax).
Oral laxatives and stool softeners may prevent constipation; rectal
sup-positories may be used if oral agents fail (Plaisance & Ellis, 2002).
INADEQUATE PAIN RELIEF
One factor commonly
associated with ineffective pain relief is an inadequate dose of opioid. This
is most likely to occur when the caregiver underestimates the patient’s pain or
the route of ad-ministration is changed without the differences in absorption
and action being considered. Consequently, the patient receives doses too small
to be effective and, possibly, too infrequently to relieve pain. For example,
if opioid delivery is changed from the intra-venous route to the oral route,
the oral dose must be approxi-mately three times greater than that given parenterally
to provide relief. Because of differences in absorption of orally administered
opioids among individuals, the patient must be assessed carefully to ensure
that the pain is relieved.
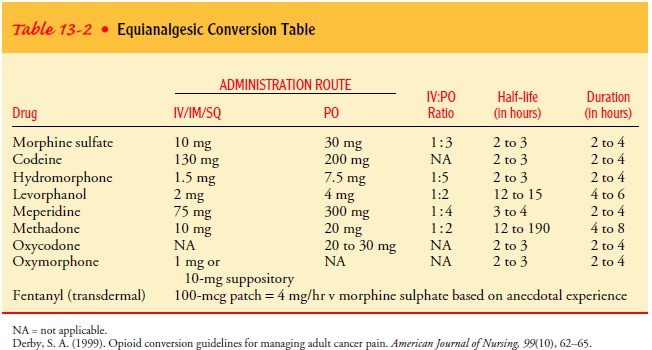
Table 13-2 lists opioids
and dosages that are equivalent to morphine. In general, no recalculation needs
to be done when switching from one brand of an agent to another brand of the
same medication, with the exception of extended-release oral morphine.
Currently, three brands of extended-release morphine (MS Contin, Oramorph,
Kadian) are commonly used by cancer patients. Although these agents come in the
same dosage form and contain the same drug, they are not considered
therapeuti-cally equivalent because they employ different release mecha-nisms.
Patients who need to switch brands should be monitored carefully both for
overdose and for inadequate pain relief.
OTHER EFFECTS OF OPIOIDS
During the health history, when asked about drug allergies, pa-tients
with previous hospital experience (especially for surgery) may report that they
are “allergic” to morphine. This report should be thoroughly investigated.
Commonly, this “allergy” will be described as itching only. Pruritus (itching)
is a frequent prob-lem associated with opioids administered through any route,
but it is not an allergic reaction. Itching can be relieved by adminis-tering
prescribed antihistamines. Epidurally administered opioids may also cause
urinary retention or pruritus. The patient should be monitored and may require
urinary catheterization. Small doses of naloxone may be prescribed to relieve
these problems in patients who are receiving epidural opioids for the relief of
acute postoperative pain.
A number of factors may
influence the safety and effectiveness of opioid administration. Opioid
analgesic agents are primarily metabolized by the liver and excreted by the
kidney. Therefore, metabolism and excretion of analgesic medications will be
im-paired in patients with liver or kidney disease, increasing the risk of cumulative
or toxic effects. In addition, normeperidine, a metabolite of meperidine, may
rapidly or unexpectedly accumu-late to toxic levels. This is more likely to
occur in patients with impaired kidney function and may result in seizures in
suscepti-ble patients.
Patients with untreated hypothyroidism are more susceptible to the
analgesic effects and side effects of opioids. In contrast, pa-tients with
hyperthyroidism may require larger doses for pain re-lief. Patients with a
decreased respiratory reserve from disease or aging may be more susceptible to
the depressant effects of opioids and must be carefully monitored for
respiratory depression.
Dehydrated patients are at increased risk for the hypotensive effects of
opioids. Patients who become hypotensive after the ad-ministration of an opioid
should be kept recumbent and rehydrated unless fluids are contraindicated.
Patients who are dehydrated are also more likely to experience nausea and
vomiting with opioid use. Rehydration usually relieves these symptoms.
Patients receiving
certain other medications, such as mono-amine oxidase (MAO) inhibitors,
phenothiazines, or tricyclic antidepressants, may have an exaggerated response
to the de-pressant effects of opioids. Patients taking these medications should
receive small doses of opioids and must be monitored closely. Continued pain in
these patients indicates that a thera-peutic level of the analgesic has not
been achieved. The patient must be monitored for sedation even if an analgesic
effect has not been obtained.
TOLERANCE AND ADDICTION
There is no maximum safe
dosage of opioids, nor is there any eas-ily identifiable therapeutic serum
level. Both the maximal safe dosage and therapeutic serum level are relative
and individual. Tolerance (the need
for increasing doses of opioids to achieve thesame therapeutic effect) will
develop in almost all patients taking opioids over an extended period. Patients
requiring opioids over a long term, especially cancer patients, will need
increasing doses to relieve pain. After the first few weeks of therapy, the
patient’s dosing requirements usually level off. Patients who become tol-erant
to the analgesic effects of large doses of morphine may ob-tain pain relief by
switching to a different opioid. Symptoms of physical dependence may occur when the opioids are discontin-ued; dependence
often occurs with opioid tolerance and does not indicate an addiction.
Addiction is a behavioral pattern of substance use character-ized by a compulsion
to take the drug primarily to experience its psychic effects. Fear that
patients will become addicted or de-pendent on opioids has contributed to
inadequate treatment of pain. This fear is commonly expressed by health care
providers as well as patients and results from lack of knowledge about the low
risk of addiction.
In an often-cited classic study (Porter & Jick, 1980) of more than
11,000 patients receiving opioids for a medical indication, only four patients
without a history of substance abuse could be identified as becoming addicted.
Addiction following therapeu-tic opioid administration is so negligible that it
should not be a consideration when caring for the patient in pain. Thus,
patients and health care providers should be dissuaded from withholding pain
medication because of concerns about addiction.
Nonsteroidal Anti-inflammatory Drugs
NSAIDs are thought to decrease pain by inhibiting cyclo-oxygenase (COX),
the rate-limiting enzyme involved in the production of prostaglandin from
traumatized or inflamed tissues. There are two types of COX: COX-1 and COX-2.
COX-1 is involved with mediating prostaglandin formation involved in the
maintenance of physiologic functions. Some of the physiologic functions
in-clude platelet aggregation through the provision of thromboxane precursors
and increased gastric mucosal blood flow. This pre-vents ischemia and promotes
mucosal integrity. Inhibition of COX-1 will result in gastric ulceration,
bleeding, and renal dam-age. The second type, COX-2, mediates prostaglandin
forma-tion that results in symptoms of pain, inflammation, and fever. Thus,
inhibition of COX-2 is desirable. Newer NSAIDs such as celecoxib (Celebrex),
rofecoxib (Vioxx), and valdecoxib (Bextra) are COX-2 inhibitors. Ibuprofen
(Advil, Motrin), another NSAID, blocks both COX-1 and COX-2 and is effec-tive
in relieving mild to moderate pain and has a low incidence of adverse effects.
Aspirin, the oldest NSAID, also blocks COX-1 as well as COX-2; however, because
it causes frequent and severe side effects, aspirin is infrequently used to
treat significant acute or chronic pain.
NSAIDs are very helpful in treating arthritic diseases and may be
especially powerful in treating cancer-related bone pain. They have been
effectively combined with opioids to treat postopera-tive and other severe
pain. The use of an NSAID with an opioid relieves pain more effectively than
the opioid alone. In such cases, the patient may obtain pain relief with less
opioid and fewer side effects. It has been shown that intraoperative
administration of NSAIDs results in improved postoperative pain control
follow-ing laparoscopic surgery and in some cases shorter hospital stays
(McLaughlin, 1994).
A regimen of a
fixed-dose, time-contingent NSAID (eg, every 4 hours) and a separately
administered fluctuating dose of opioid may be effective in managing moderate
to severe cancer pain. In more severe pain, the opioid dose will also be fixed,
with an addi-tional fluctuating dose as needed for breakthrough pain (a sud-den increase in pain despite the
administration of pain-relieving medications). These regimens result in better
pain relief with fewer opioid-related side effects.
Most patients tolerate NSAIDs well. However, those with im-paired kidney
function may require a smaller dose and must be monitored closely for side
effects. Patients taking NSAIDs bruise easily because NSAIDs have some
anticoagulant effect. More-over, they may displace other medications, such as
warfarin (Coumadin), from serum proteins and increase their effects. High doses
or prolonged use can irritate the stomach and in some cases result in
gastrointestinal bleeding as well. Thus, monitoring the patient for
gastrointestinal bleeding is indicated.
Gerontologic
Considerations Related to Analgesic Agents
Physiologic changes in older adults require that analgesic agents be
administered with caution. Drug interactions are more likely to occur in older
adults because of the higher incidence of chronic illness and the increased use
of prescription and over-the-counter medications. Although the elderly
population is an extremely het-erogeneous group, differences in response to
pain or medications by a patient in this 40-year span (60 to 100 years) are
more likely to be due to chronic illness or other individual factors than age.
Before administering opioid and nonopioid analgesic agents to elderly patients,
the nurse needs to obtain a careful medication history to identify potential
drug interactions.
Absorption and metabolism of medications are altered in elderly patients
because of decreased liver, renal, and gastrointestinal func-tion. In addition,
changes in body weight, protein stores, and dis-tribution of body fluid alter
the distribution of medications in the body. As a result, medications are not
metabolized as quickly and blood levels of the medication remain higher for a
longer period. Elderly patients are more sensitive to medications and at an
in-creased risk for drug toxicity (American Geriatrics Society, 1998).
Opioid and nonopioid analgesic medications can be given ef-fectively to
elderly patients but must be used cautiously because of the increased
susceptibility to depression of both the nervous and the respiratory systems.
Although there is no reason to avoid opioids simply because a person is
elderly, meperidine should be avoided because its active and neurotoxic
metabolite, normeperi-dine, is more likely to accumulate in the elderly. In
addition, be-cause of decreased binding of meperidine by plasma proteins, blood
concentrations of the medication twice those found in younger patients may
result.
In many cases, the initial dose of analgesic medication prescribed for
an elderly patient may be the same as that for a younger person, or slightly
smaller than the normal dose, but because of slowed me-tabolism and excretion
related to aging, the safe interval for subse-quent doses may be longer (or
prolonged). As always, the best guide to pain management and administration of
analgesic agents in all patients regardless of age is what the patient says.
The elderly pa-tient may obtain more pain relief for a longer time than a
younger patient. As a result, smaller, less frequent doses may be required. The
American Geriatrics Society (2002) has published clinical prac-tice guidelines
for managing chronic pain in elderly patients.
Tricyclic Antidepressant Agents and Anticonvulsant Medications
Pain of neurologic origin (eg, causalgia, tumor impingement on a nerve,
postherpetic neuralgia) is difficult to treat and in general is not responsive
to opioid therapy. When these pain syndromes are accompanied by dysesthesia (burning
or cutting pain), they may be responsive to a tricyclic antidepressant or an
antiseizure agent. When indicated, tricyclic antidepressant agents, such as
amitriptyline (Elavil) or imipramine (Tofranil), are prescribed in doses
considerably smaller than those generally used for depres-sion. The patient
needs to know that a therapeutic effect may not occur before 3 weeks.
Antiseizure medications such as phenytoin (Dilantin) or carbamazepine
(Tegretol) also are used in doses lower than those prescribed for seizure
disorders. Because a vari-ety of medications can be tried, the nurse should be
familiar with the possible side effects and should teach the patient and family
how to recognize these effects.
Related Topics