Chapter: Medical Surgical Nursing: Pain Management
Pain Transmission - Pathophysiology of Pain
Pathophysiology of Pain
The sensory experience
of pain depends on the interaction be-tween the nervous system and the
environment. The processing of noxious stimuli and the resulting perception of
pain involve the peripheral and central nervous systems.
PAIN
TRANSMISSION
Among the nerve
mechanisms and structures involved in the transmission of pain perceptions to
and from the area of the brain that interprets pain are nociceptors, or pain
receptors, and chem-ical mediators. Nociceptors
are receptors that are preferentially sensitive to a noxious stimulus.
Nociceptors are also called pain receptors, but the former term is preferred.
Nociceptors
Nociceptors are free nerve endings in the skin that respond only to
intense, potentially damaging stimuli. Such stimuli may be me-chanical,
thermal, or chemical in nature. The joints, skeletal mus-cle, fascia, tendons,
and cornea also have nociceptors that have the potential to transmit stimuli
that produce pain. However, the large internal organs (viscera) do not contain
nerve endings that respond only to painful stimuli. Pain originating in these
organs results from intense stimulation of receptors that have other pur-poses.
For example, inflammation, stretching, ischemia, dilation, and spasm of the
internal organs all cause an intense response in these multipurpose fibers and
can cause severe pain.
Nociceptors are part of
complex multidirectional pathways. These nerve fibers branch very near their
origin in the skin and send fibers to local blood vessels, mast cells, hair
follicles, and sweat glands. When these fibers are stimulated, histamine is
re-leased from the mast cells, causing vasodilation. Nociceptors respond to
high-intensity mechanical, thermal, and chemical stimuli. Some receptors
respond to only one type of stimuli; others, called polymodal nociceptors,
respond to all three types of stimuli. These highly specialized neurons
transfer the mechani-cal, thermal, or chemical stimulus into electrical
activity or action potentials.
The cutaneous fibers
located more centrally further branch and communicate with the paravertebral
sympathetic chain of the nervous system and with large internal organs. As a
result of the connections between these nerve fibers, pain is often
accom-panied by vasomotor, autonomic, and visceral effects. In a patient with
severe acute pain, for example, gastrointestinal peristalsis may decrease or
stop.
Peripheral Nervous System
A number of algogenic
(pain-causing) substances that affect the sensitivity of nociceptors are
released into the extracellular tissue as a result of tissue damage. Histamine,
bradykinin, acetylcholine, serotonin, and substance P are chemicals that
increase the trans-mission of pain. The transmission of pain is also referred
to as nociception. Prostaglandins are chemical substances
thought toincrease the sensitivity of pain receptors by enhancing the
pain-provoking effect of bradykinin. These chemical mediators also cause
vasodilation and increased vascular permeability, resulting in redness, warmth,
and swelling of the injured area.
Once nociception is
initiated, the nociceptive action poten-tials are transmitted by the peripheral
nervous system (Porth, 2002). The first-order neurons travel from the periphery
(skin, cornea, visceral organs) to the spinal cord via the dorsal horn. There
are two main types of fibers involved in the transmission of nociception.
Smaller, myelinated Aδ (A delta) fibers
transmit nociception rapidly, which produces the initial “fast pain.” Type C
fibers are larger, unmyelinated fibers that transmit what is called second
pain. This type of pain has dull, aching, or burning qualities that last longer
than the initial fast pain. The type and concentration of nerve fibers to
transmit pain vary by tissue type.
If there is repeated C fiber input, a greater response is noted in
dorsal horn neurons, causing the person to perceive more pain. In other words,
the same noxious stimulus produces hyperalge-sia, and the person reports
greater pain than was felt at the first stimulus. For this reason, it is
important to treat patients with analgesic agents when they first feel the
pain. Patients require less medication and experience more effective pain
relief if analgesia is administered before the patient becomes sensitized to
the pain.
Chemicals that reduce or inhibit the transmission or perception of pain include endorphins and enkephalins. These morphine-like neurotransmitters are endogenous (produced by the body). They are examples of substances that reduce nociceptive trans-mission when applied to certain nerve fibers. The term “endor-phin” is a combination of two words: endogenous and morphine. Endorphins and enkephalins are found in heavy concentrations in the central nervous system, particularly the spinal and medullary dorsal horn, the periaqueductal gray matter, hypothalamus, and amygdala. Morphine and other opioid medications act at recep-tor sites to suppress the excitation initiated by noxious stimuli. The binding of opioids to receptor sites is responsible for the effects noted after their administration. Each receptor (mu, kappa, delta) responds differently when activated. Table 13-1 summarizes the classification and action of opioid receptors.
Central Nervous System
After tissue injury occurs, nociception (the neurologic transmis-sion of
pain impulses) to the spinal cord via the Aδ and C fibers continues. The fibers enter the dorsal horn, which is
divided into laminae based on cell type. The laminae II cell type is commonly
referred to as the substantia gelatinosa. In the substantia gelati-nosa are
projections that relay nociception to other parts of the spinal cord (Fig.
13-3).Nociception continues from the spinal cord to the reticular formation,
thalamus, limbic system, and cerebral cortex. Here nociception is localized and
its characteristics become apparent to the person, including the intensity. The
involvement of the reticular formation, limbic, and reticular activating
systems is re-sponsible for the individual variations in the perception of
nox-ious stimuli. Individuals may report the same stimulus differently based on
their anxiety, past experiences, and expectations. This is a result of the
conscious perception of pain.
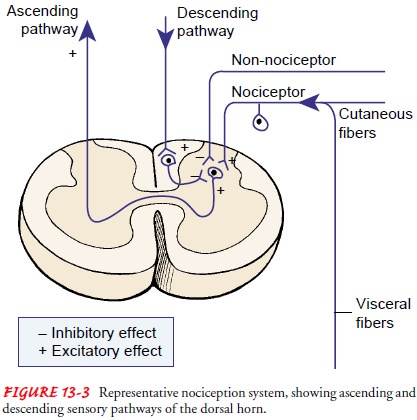
For pain to be
consciously perceived, neurons in the ascend-ing system must be activated.
Activation occurs as a result of input from the nociceptors located in the skin
and internal or-gans. Once activated, the inhibitory interneuronal fibers in
the dorsal horn inhibit or turn off the transmission of noxious stim-ulating
information in the ascending pathway.
Descending Control System
The descending control system is a system of fibers that originate in
the lower and midportion of the brain (specifically the peri-aqueductal gray
matter) and terminate on the inhibitory inter-neuronal fibers in the dorsal
horn of the spinal cord. This system is probably always somewhat active; it
prevents continuous trans-mission of stimuli as painful, partly through the
action of the en-dorphins. As nociception occurs, the descending control system
is activated to inhibit pain.
Cognitive processes may stimulate endorphin production in the descending control system. The effectiveness of this system is illustrated by the effects of distraction. The distractions of visitors or a favorite TV show may increase activity in the descending control system. Therefore, the person who has visitors may not report pain because activation of the descending control system results in less noxious or painful information being transmitted to consciousness. Once the distraction by the visitors ends, activ-ity in the descending control system decreases, resulting in in-creased transmission of painful stimuli.
The interconnections between the descending neuronal sys-tem and the
ascending sensory tract are called inhibitory inter-neuronal fibers. These
fibers contain enkephalin and are primarily activated through the activity of non-nociceptor peripheral fibers
(fibers that normally do not transmit painful or noxious stimuli) in the same
receptor field as the pain receptor, and descending fibers, grouped together in
a system called descending control. The enkephalins and endorphins are thought
to inhibit pain im-pulses by stimulating the inhibitory interneuronal fibers,
which in turn reduce the transmission of noxious impulses via the as-cending
system (Puig & Montes, 1998).
The classic gate control theory of pain, described by Melzack and Wall
in 1965, was the first to clearly articulate the existence of a pain-modulating
system (Melzack, 1996). This theory pro-poses that stimulation of the skin
evokes nervous impulses that are then transmitted by three systems located in
the spinal cord. The substantia gelatinosa in the dorsal horn, the dorsal
column fibers, and the central transmission cells act to influence nocicep-tive
impulses. The noxious impulses are influenced by a “gating mechanism.” Melzack
and Wall proposed that stimulation of the large-diameter fibers inhibits the
transmission of pain, thus “clos-ing the gate.” Conversely, when smaller fibers
are stimulated, the gate is opened. The gating mechanism is influenced by nerve
impulses that descend from the brain. This theory proposes a spe-cialized
system of large-diameter fibers that activate selective cog-nitive processes
via the modulating properties of the spinal gate. Figure 13-4 shows a schematic
representation of a gate control system and nociceptive pathways.
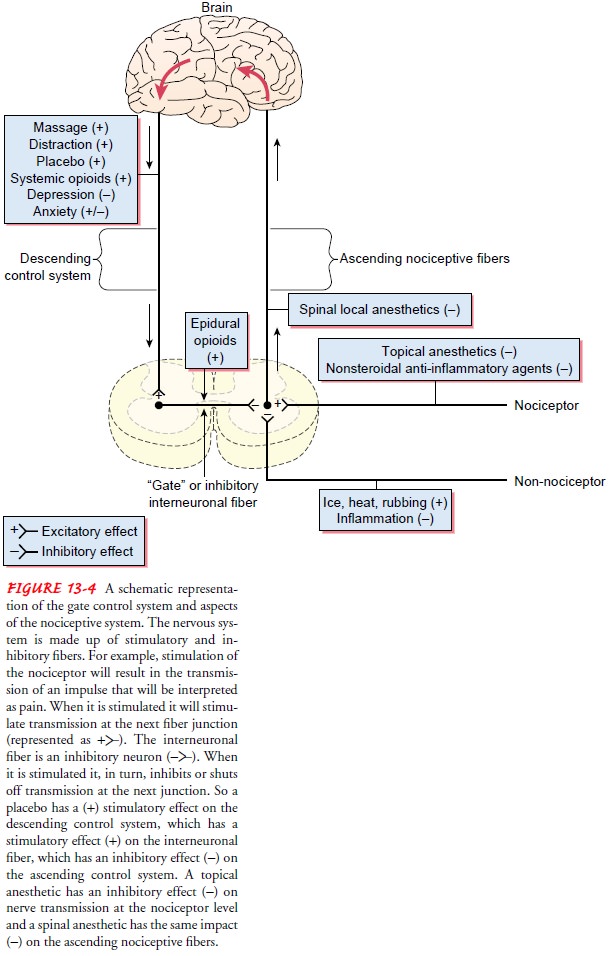
The gate control theory was important because it was the first theory to
suggest that psychological factors play a role in the per-ception of pain. The
theory guided research toward the cognitive-behavioral approaches to pain
management. This theory helps to explain how interventions such as distraction
and music therapy provide pain relief.
Melzack (1996) extended the gate control theory after care-fully analyzing phantom limb pain. He proposed that a large, widespread network of neurons exists that consists of loops be-tween the thalamus and cortex and between the cortex and the limbic system. Melzack labeled this network the neuromatrix.
As information is processed
in the neuromatrix, a characteristic pat-tern emerges. This pattern, referred
to as the neurosignature, is a continuous outflow from the neuromatrix.
Ultimately, the neuro-signature output, with a constant stream of input and
varying patterns, produces the feelings of the whole body with constantly
changing qualities.
Melzack (1996) theorized that in the absence of modulating inputs from
the missing limb, the active neuromatrix produces a neurosignature pattern that
is perceived as pain. The neuro-matrix theory highlights the role of the brain
in sustaining the experience of pain. Some researchers have criticized this
theory as not adding to the understanding of how psychological factors
influence pain (Keefe, Lefebvre & Starr, 1996). While the neuro-matrix
theory might explain unusual pain phenomena, its con-tribution to understanding
pain management remains to be seen.
Related Topics