Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Vaccines
Pharmaceutical Aspects of Vaccines
PHARMACEUTICAL ASPECTS
Production
Except for synthetic peptides, vaccines are derived from microorganisms or from animal cells. For optimal expression of the required vaccine component(s), these microorganisms or animal cells can be genetically modified. Animal cells are used for the cultivation of viruses and for the production of some subunit vaccine components, and have the advantage that the vaccine components are released into the culture medium.
Three stages can be discerned in the manufac-ture of cell-derived vaccines: (1) cultivation or up-stream processing, (2) purification or downstream processing, and (3) formulation. Whereas the formulation is addressed in the following section.
Formulation
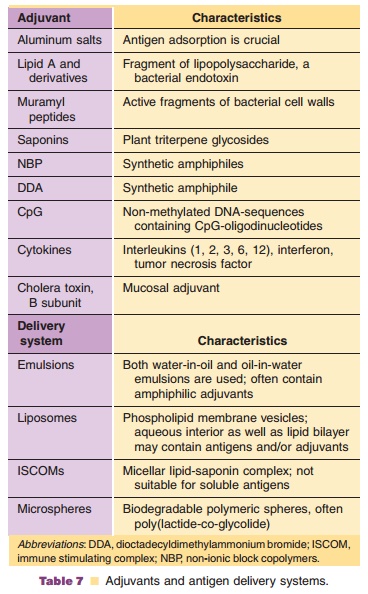
Adjuvants and Delivery Systems
The success of immunization is not only dependent on the nature of the immunogenic components, but also on their presentation form. Therefore, the search for effective and acceptable adjuvants (Schijns, 2000) and delivery systems (Kersten and Hirschberg, 2004) is an important issue in modern vaccine development
Adjuvants are defined as any material that can increase the humoral and cellular response against an antigen. Colloidal aluminum salts (hydroxide,phosphate) are widely used in many classical vaccine formulations. Other adjuvants are in experimental testing or are used in veterinary vaccines. Delivery systems are injectable devices that allow multimeric presentation of antigens. They can also contain adjuvants. Table 7 shows a list of some well-known adjuvants and delivery systems.
Adjuvants and delivery systems stimulate the immune system by several mechanisms of action (Schijns, 2000):
1. A depot effect leading to slow antigen release and prolonged antigen presentation.
2. Attraction and stimulation of APCs by some local tissue damage and binding to PRRs present on APCs.
3. Delivery of the antigen to regional lymph nodes by improved antigen uptake, transport and pre-sentation by APCs.
A particular adjuvant may act by one or more of these mechanisms
Combination Vaccines
Since oral immunization is not possible for most available vaccines (see section “Route of Administration,” above), the strategy to mix indivi-dual vaccines in order to limit the number of injections has been common practice since many years. Currently, vaccines are available containing up to six non-related antigens: diphtheria-tetanus-pertussis-hepatitis B-polio-Haemophilus influenzae type b vaccine. Another example is MMR vaccine, alone or in combination with varicella vaccine. Sometimes a vaccine contains antigens from several subtypes of a particular pathogen. Heptavalent pneumococcal vac-cine is an example. This vaccine contains polysacchar-ides from seven pneumococcal strains, conjugated to a carrier protein to improve immunogenicity.
Combining vaccine components sometimes results in pharmaceutical as well as immunological problems. For instance, formaldehyde-containing components may chemically react with other components; an unstable antigen may need freeze drying whereas other antigens cannot be frozen. Components that are not compatible can be mixed prior to injection, if there is no short-term incompatibility. To this end, dual-chamber syringes have been developed.
From an immunological point of view, the immunization schedules of the individual compo-nents of combination vaccines should match. Even when this condition is fulfilled and the components are pharmaceutically compatible, the success of a combination vaccine is not warranted. Vaccine com-ponents in combination vaccines may exhibit a different behavior in vivo compared to separate administration of the components. For instance, enhancement (Paradiso et al., 1993) as well as suppression (Gold et al., 1994) of humoral immune responses has been reported.
Characterization
Second and third generation conventional vaccines and modern vaccines are well-defined products in terms of immunogenicity, structure, and purity. This means that the products can be characterized with a combination of appropriate biochemical, physico-chemical, and immunochemical techniques . Vaccines have to meet the same standards as other biotechnological pharmaceuticals. The use of modern analytical techniques for the design and release of new vaccines is gaining importance. Currently, animal experiments are needed for quality control of many vaccines but in vitro analytical techniques may eventually partly substitute preclini-cal tests in vivo. During the development of the production process of a vaccine component, a combination of suitable assays can be defined. These assays can subsequently be applied during its routine production.
Column chromatographic (HPLC) and electro-phoretic techniques like gel electrophoresis and capillary electrophoresis provide information about the purity, molecular weight, and electric charge of the vaccine component. Physico-chemical assays com-prise mass spectrometry, nuclear magnetic resonance spectroscopy, and light spectroscopy, including circu-lar dichroism and fluorescence spectroscopy. Information is obtained mainly about the molecular weight and the conformation of the vaccine compo-nent. Immunochemical assays, such as enzyme-linked immunoassays and radioimmunoassays, are powerful methods for the quantification of the vaccine compo-nent. By using well-defined monoclonal antibodies (preferably with the same specificity as those of protective human antibodies) information can be obtained about the conformation and accessibility of the epitope to which the antibodies are directed. Moreover, the use of biosensors makes it possible to measure antigen-antibody interactions momentarily, allowing accurate determination of binding kinetics and affinity constants.
Storage
Depending on their specific characteristics, vaccines are stored as solution or as a freeze-dried formulation, usually at 2LC to 8LC. Their shelf-life depends on the composition and physico-chemical characteristics of the vaccine formulation and on the storage conditions, and typically is in the order of several years. The quality of the container can influence the long-term stability of vaccines, e.g., through adsorption or pH changes resulting from contact with the vial wall. The use of pH indicators or temperature or time sensitive labels (“vial vaccine monitors,” which change color when exposed to extreme temperatures or after the expiration date) can avoid unintentional administra-tion of inappropriately stored or expired vaccine.
Related Topics