Chapter: Medical Surgical Nursing: Management of Patients With Oncologic or Degenerative Neurologic Disorders
ParkinsonŌĆÖs Disease
PARKINSONŌĆÖS DISEASE
ParkinsonŌĆÖs disease is a
slowly progressing neurologic movement disorder that eventually leads to
disability. The degenerative or id-iopathic form is the most common; there is
also a secondary form with a known or suspected cause. Although the cause of
most cases is unknown, research suggests several causative factors, including
ge-netics, atherosclerosis, excessive accumulation of oxygen free radi-cals,
viral infections, head trauma, chronic antipsychotic medication use, and some
environmental exposures. Parkinsonian symptoms usually first appear in the
fifth decade of life; however, cases have been diagnosed at the age of 30
years. It is the fourth most common neurodegenerative
disease. ParkinsonŌĆÖs disease affects men morefrequently than women and
nearly 1% of the population older than 60 years of age (Gray & Hildebrand,
2000).
Pathophysiology
ParkinsonŌĆÖs disease is associated with decreased levels of dopamine due to destruction of pigmented neuronal cells in the substantia nigra in the basal ganglia of the brain (Fig. 65-4). The nuclei of the substantia nigra project fibers or neuronal pathways to the corpus striatum, where neurotransmitters are key to control of complex body movements.
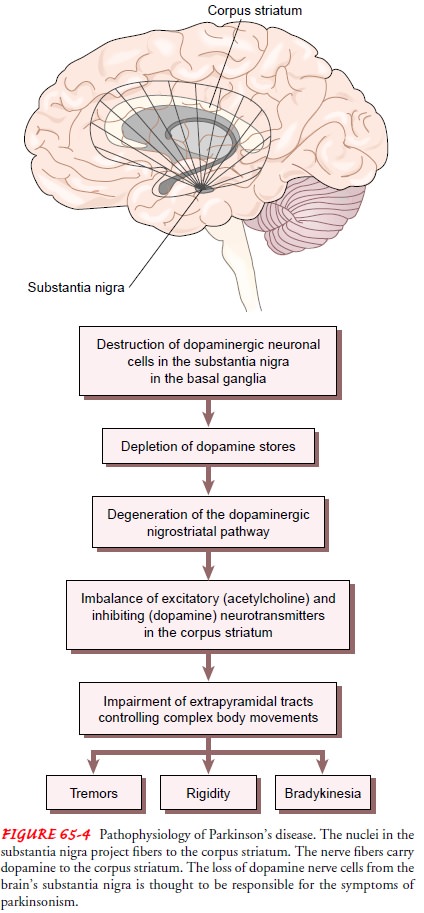
Through the
neurotransmitters acetyl-choline (excitatory) and dopamine (inhibitory),
striatal neurons relay messages to the higher motor centers that control and
refine motor movements. The loss of dopamine stores in this area of the brain
results in more excitatory neurotransmitters than inhibitory neurotransmitters,
leading to an imbalance that affects voluntary movement.
Basic science research in the past two decades has
revealed that more neurotransmitter pathways in the brain than just the
dopaminergic system are involved. Parts of the glutamatergic, cholinergic,
tryptaminergic, noradrenergic, adrenergic, seroton-ergic, and peptidergic
pathways (responsible for cell metabolism, growth, nutrition, and so forth)
show damage in ParkinsonŌĆÖs dis-ease (Chase, Oh & Konitsiotis, 2000;
Przuntek, 2000; Rascol, 2000).
Clinical symptoms do not appear until 60% of the
pigmented neurons are lost and the striatal dopamine level is decreased by 80%.
Cellular degeneration impairs the extrapyramidal tracts that control
semiautomatic functions and coordinated movements; motor cells of the motor cortex
and the pyramidal tracts are not affected.
Clinical Manifestations
ParkinsonŌĆÖs disease has
a gradual onset and symptoms progress slowly over a chronic, prolonged course.
The three cardinal signs are tremor, rigidity, and bradykinesia (abnormally slow move-ments). Other features include
hypokinesia, gait disturbances, and postural instability (Gray &
Hildebrand, 2000).
TREMOR
Although symptoms are
variable, a slow, unilateral, resting tremor is present in 70% of patients at
the time of diagnosis. Resting tremor characteristically disappears with
purposeful movement but is evident when the extremities are motionless. The
tremor may present as a rhythmic, slow turning motion (pronationŌĆō supination)
of the forearm and the hand and a motion of the thumb against the fingers as if
rolling a pill (Fig. 65-5). Tremor is present while the patient is at rest; it
increases when the patient is walking, concentrating, or feeling anxious.
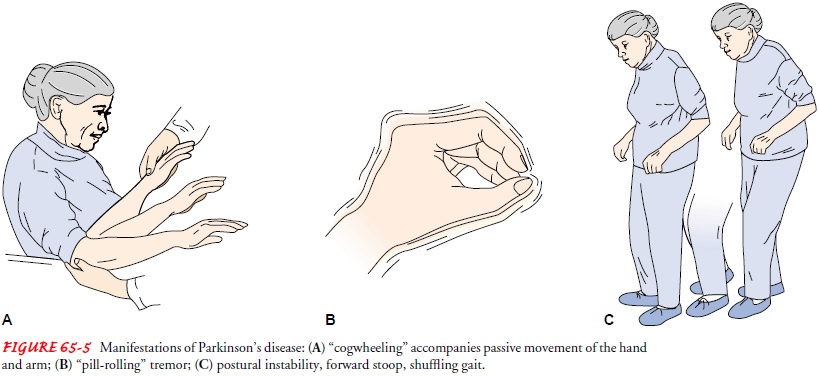
RIGIDITY
Resistance to passive
limb movement characterizes muscle rigidity. Passive movement of an extremity
may cause the limb to move in jerky increments referred to as cogwheeling.
Rigidity of the pas-sive extremity increases when another extremity is engaged
in voluntary active movement. Stiffness of the neck, trunk, and shoul-ders is
common. Early in the disease, the patient may complain of shoulder pain.
BRADYKINESIA
One of the most common features of ParkinsonŌĆÖs disease is
brady-kinesia. Patients take longer to complete most activities and have
difficulty initiating movement, such as rising from a sitting posi-tion or
turning in bed.
Hypokinesia (abnormally
diminished movement) is also com-mon and may appear after the tremor. The
freezing phenomenon is a transient inability to perform active movement and is
thought to be an extreme form of bradykinesia. Additionally, the patient tends
to shuffle and exhibits a decreased arm swing. As dexterity declines, micrographia (shrinking, slow
handwriting) develops. The face becomes increasingly masklike and
expressionless and the frequency of blinking decreases. Dysphonia (soft, slurred, low-pitched, and less audible speech) may
occur due to weakness and incoordination of the muscles responsible for speech.
In many cases, the patient develops dysphagia, begins to drool, and is at risk
for choking and aspiration.
The patient commonly develops postural and gait problems. There is a loss of postural reflexes, and the patient stands with the head bent forward and walks with a propulsive gait. The posture is caused by the forward flexion of the neck, hips, knees, and el-bows. The patient may walk faster and faster, trying to move the feet forward under the bodyŌĆÖs center of gravity (shuffling gait). Difficulty in pivoting and loss of balance (either forward or back-ward) places the patient at risk for falls.
OTHER MANIFESTATIONS
The effect of ParkinsonŌĆÖs disease on the basal ganglia
often pro-duces autonomic symptoms that include excessive and un-controlled
sweating, paroxysmal flushing, orthostatic hypotension, gastric and urinary
retention, constipation, and sexual distur-bances (Herndon et al., 2000).
Psychiatric changes are
often interrelated and may be predictive of one another. They include
depression, dementia (progressive
mental deterioration), sleep disturbances, and hallucinations (Herndon et al.,
2000). Depression is common; whether it is a reaction to the disorder or is
related to a biochemical abnormal-ity remains a question. Mental changes may
appear in the form of cognitive, perceptual, and memory deficits, although
intellect is not usually affected. A number of psychiatric manifestations
(personality changes, psychosis, dementia, and acute confusion) are common
among the elderly. The prevalence of dementia is about 25% and the pattern is
similar to that of patients with AlzheimerŌĆÖs disease. Although there is no
direct documented causal relationship, the rates of depression and dementia are
highly cor-related in these patients (Herndon et al., 2000).Approximately 41%
of women and 25% of men with Parkin-sonŌĆÖs disease experience sleep
disturbances. This may be connected to depression, dementia, or medications.
Auditory and visual hal-lucinations have been reported in approximately 37% of
persons with ParkinsonŌĆÖs and may be associated with depression, demen-tia, lack
of sleep, or adverse effects of medications (Herndon et al., 2000).
Complications associated
with ParkinsonŌĆÖs disease are com-mon and are typically related to disorders of
movement. As the disease progresses, patients are at risk for respiratory and
urinary tract infection, skin breakdown, and injury from falls. The ad-verse
effects of medications used to treat the symptoms are asso-ciated with numerous
complications.
Assessment and Diagnostic Findings
Laboratory tests and imaging studies are not helpful in
the diag-nosis of ParkinsonŌĆÖs disease, although PET scanning has been used in
evaluating levodopa (precursor of dopamine) uptake and conversion to dopamine
in the corpus striatum (Freed et al., 2001). Currently, the disease is
diagnosed clinically from the pa-tientŌĆÖs history and the presence of two of the
three cardinal man-ifestations: tremor, muscle rigidity, and bradykinesia.
Early diagnosis can be
difficult because the patient can rarely pinpoint when symptoms started. Often
a family member notices a change such as stooped posture, a stiff arm, a slight
limp, tremor, or slow, small handwriting. The medical history, presenting
symp-toms, neurologic examination, and response to pharmacologic management are
carefully evaluated when making the diagnosis.
Medical Management
Treatment is directed at
controlling symptoms and maintaining functional independence because there are
no medical or surgical approaches that prevent disease progression. Care is
individual-ized for each patient based on presenting symptoms and social,
occupational, and emotional needs. Pharmacologic management is the mainstay of
treatment, although advances in research have led to increased interest in
surgical interventions. Patients are usu-ally cared for at home and admitted to
the hospital only for com-plications or to initiate new treatments.
PHARMACOLOGIC THERAPY
Antiparkinsonian
medications act by 1) increasing striatal dopa-minergic activity, 2) reducing
the excessive influence of excitatory cholinergic neurons on the extrapyramidal
tract, thereby restor-ing a balance between dopaminergic and cholinergic
activities, oracting on neurotransmitter pathways other than the dopami-nergic
pathway.
Antiparkinsonian Medications.
Levodopa (Dopar,
Larodopa) isthe most effective agent and the mainstay of treatment (Karch,
2002; Obeso et al., 2001). Because levodopa is thought to pre-cipitate
oxidation, which further damages the substantia nigra and eventually speeds
disease progression, physicians delay pre-scribing the medication or increasing
the dosage for as long as possible (Karch, 2002). Levodopa is converted to
dopamine in the basal ganglia, producing symptom relief. The beneficial
ef-fects of levodopa are most pronounced in the first few years of treatment.
Benefits begin to wane and adverse effects become more severe over time.
Confusion, hallucinations, depression, and sleep alterations are associated
with prolonged use. Levodopa is usually given in combination with carbidopa
(Sinemet), an amino acid decarboxylase
inhibitor that helps to maximize the beneficial effects of levodopa by
preventing its breakdown out-side the brain and reducing its adverse effects
(Karch, 2002).
Within 5 to 10 years, most patients develop a response to
the medication characterized by dyskinesia
(abnormal involun-tary movements), including facial grimacing, rhythmic jerking
movements of the hands, head bobbing, chewing and smacking movements, and
involuntary movements of the trunk and extrem-ities. The patient may experience
an onŌĆōoff syndrome in which sudden periods of near immobility (ŌĆ£off effectŌĆØ)
are followed by a sudden return of effectiveness (ŌĆ£on effectŌĆØ). Various
adjunctive therapies are used to minimize dyskinesias (Przuntek, 2000; Rascol,
2000).
Budipine, available in
Europe but not the United States, is a non-dopaminergic, antiparkinsonian
medication that significantly reduces akinesia, rigidity, and tremor. It is
non-dopaminergic be-cause the action appears to be on neurotransmitter pathways
other than the dopaminergic pathway. It may be used as monotherapy or in
conjunction with other available antiparkinsonian medica-tions (Przuntek, 2000;
Przuntek et al., 2002). The usual dose of 40 to 60 mg is reached gradually.
Nausea and dry mouth are the most common side effects, although 75% of patients
experienced no side effects in clinical drug trials (Przuntek, 2000).
Anticholinergic Therapy.
Anticholinergic agents
(trihexyphenidyl,cycrimine, procyclidine, biperiden, and benztropine mesylate)
are effective in controlling tremor and rigidity. They may be used in
combination with levodopa. They counteract the action of the neurotransmitter
acetylcholine. Because the side effects include blurred vision, flushing, rash,
constipation, urinary retention, and acute confusional states, these
medications are often poorly tol-erated in elderly patients. Intraocular
pressure must be closely monitored: these medications are contraindicated in
patients with narrow-angle glaucoma. Patients with prostate hyperplasia are
monitored for signs of urinary retention.
Antiviral Therapy.
Amantadine hydrochloride
(Symmetrel) is anantiviral agent used in early ParkinsonŌĆÖs treatment to reduce
rigidity, tremor, and bradykinesia. It is thought to act by releasing dopamine
from neuronal storage sites. Studies suggest it may also have antiglutamatergic
properties that affect the glutamatergic pathway, thus improving
levodopa-induced dyskinesias (Rascol, 2000). Amantadine has a low incidence of
side effects, which in-clude psychiatric disturbances (mood changes, confusion,
depres-sion, hallucinations), lower extremity edema, nausea, epigastric
distress, urinary retention, headache, and visual impairment.
Dopamine Agonists.
Bromocriptine mesylate
and pergolide (ergotderivatives) are dopamine receptor agonists and are useful
in postponing the initiation of carbidopa or levodopa therapy. Dopamine
agonists are often added to the medication regimen when carbidopa or levodopa
loses effectiveness. Pergolide (Permax) is 10 times more potent than
bromocriptine mesylate (Parlodel), although this provides no therapeutic
advantage. Adverse re-actions to these medications include nausea, vomiting,
diar-rhea, lightheadedness, hypotension, impotence, and psychiatric effects.
Two new dopamine
agonists, ropinirole hydrochloride (Requip) and pramipexole (Mirapex) (nonergot
derivatives), are primarily for patients in the early stages of ParkinsonŌĆÖs
disease and are not expected to have the potentially serious adverse effects of
per-golide and bromocriptine mesylate. Pramipexole (Mirapex) canbe used without
levodopa for treatment of early disease and with levodopa in advanced stages.
Cabergoline (Dostinex), an ergot alkaloid with a long duration of action, has
been approved for use.
Monoamine Oxidase Inhibitors (MAO Inhibitors).
Of
the MAOinhibitors, selegiline (Eldepryl) is one of the most exciting and
controversial developments in the pharmacotherapy of Parkin-sonŌĆÖs disease
(Herndon et al., 2000). This medication inhibits dopamine breakdown and is
thought to slow the progression of the disease. Researchers believe this
medication may have a neuro-protective effect in the early stages of
ParkinsonŌĆÖs disease, but this has not been shown in clinical trials. Selegiline
is currently used in combination with a dopamine agonist to delay the use of
car-bidopa or levodopa therapy. Adverse effects are similar to those of
levodopa.
Catechol-O-methyltransferase (COMT) Inhibitors.
Clinical trialssuggest
that the COMT inhibitors entacapone (Comtess) and tolcapone (Tasmar) have
little effect on parkinsonian symptoms when given alone but can increase the
duration of action of car-bidopa or levodopa when given in combination with
them. COMT inhibitors block an enzyme that metabolizes levodopa, making more
levodopa available for conversion to dopamine in the brain. Entacapone and
tolcapone reduce motor fluctuations in patients with advanced ParkinsonŌĆÖs
disease.
Antidepressants.
Tricyclic antidepressants may be prescribed
toalleviate the depression that is so common in ParkinsonŌĆÖs disease. The usual
dosage is one-third to one-half the dosage used in de-pressed patients without
ParkinsonŌĆÖs disease. Amitriptyline is typ-ically prescribed because of its
anticholinergic and antidepressant effect. Serotonin reuptake inhibitors, such
as fluoxetine hydro-chloride (Prozac) and bupropion hydrochloride (Wellbutrin),
are effective for treating depression but may aggravate parkinsonism.
Antihistamines.
Diphenhydramine hydrochloride
(Benadryl),orphenadrine citrate (Banflex), and phenindamine hydrochloride
(Neo-Synephrine) have mild central anticholinergic and sedative effects and may
reduce tremors.
SURGICAL MANAGEMENT
The limitations of
levodopa therapy, improvements in stereotac-tic surgery, and new approaches in
transplantation have renewed interest in the surgical treatment of ParkinsonŌĆÖs
disease. In pa-tients with disabling tremor, rigidity, or severe
levodopa-induced dyskinesia, surgery may be considered. Although surgery
provides some relief in selected patients, it has not been shown to alter the
course of the disease or produce permanent improvement.
Stereotactic Procedures.
Thalamotomy and
pallidotomy areeffective in relieving many of the symptoms of ParkinsonŌĆÖs
dis-ease. Patients eligible for these procedures are those who have had an
inadequate response to medical therapy; they must meet strict criteria to be
eligible. Candidates eligible for these procedures are patients with idiopathic
ParkinsonŌĆÖs disease who are taking max-imum doses of antiparkinsonian
medications. Patients with de-mentia and atypical ParkinsonŌĆÖs disease are
usually not considered for stereotactic procedures. ParkinsonŌĆÖs disease rating
scales and specific neurologic testing are used to identify eligible
patients.The intent of thalamotomy and pallidotomy is to interrupt the nerve
pathways and thereby alleviate tremor or rigidity. During thalamotomy, a
stereotactic electrical stimulator destroys part of the ventrolateral portion
of the thalamus in an attempt to reduce tremor; the most common complications
are ataxia and hemiparesis. Pallidotomy involves destroying part of the ventral
aspect of the medial globus pallidus through electrical stimula-tion in
patients with advanced disease. The procedure is effective in reducing
rigidity, bradykinesia, and dyskinesia, thus improv-ing motor function and
activities of daily living in the immediate postoperative course. In small
studies, clinical improvements have been demonstrated over 3 to 4 years. The
clinical benefit is greater in patients younger than 60 years (Freed et al.,
2001). Complications include hemiparesis, stroke, and visual changes.
CT, x-rays, MRI, or angiography is used to localize the
ap-propriate surgical site in the brain. Then the patientŌĆÖs head is po-sitioned
in a stereotactic frame (Fig. 65-6). The surgeon makes an incision in the skin
and then a burr hole. Next, the surgeon passes an electrode through the burr
hole to the target area in the thalamus or globus pallidum. The desired
response of the patient to the electrical stimulation is the basis for the
final site chosen by the neurosurgeon. Stereotactic procedures are completed on
one side of the brain at a time. If rigidity or tremor is bilateral, a 6-month
interval is suggested between procedures.
Neural Transplantation.
Surgical implantation of adrenal medul-lary tissue
into the corpus striatum is performed in an effort to reestablish normal
dopamine release. Preliminary evidence has shown high morbidity and mortality
rates, and the implants ap-pear to improve parkinsonian symptoms for only 6
months. Re-searchers are conducting studies to determine if transplanting human
fetal brain cells or genetically engineered cells into the ni-grostriatal
region is effective (Aminoff, 2000). Legal and ethical issues surrounding the
use of fetal brain cells have limited the im-plementation of this procedure.
Recently, fetal pig neuronal cells survived transplantation into a patient with
ParkinsonŌĆÖs dis-ease; this may provide an alternative to human cell transplants
(Aminoff, 2000).
Deep Brain Stimulation.
Recently approved by the FDA,pacemaker-like brain implants show promising results in relieving tremors. The stimulation can be bilateral or unilateral, although bilateral stimulation of the subthalamic nucleus is thought to be of greater benefit to patients than results achieved with thala-motomy, pallidotomy, or fetal nigral transplantation (Obeso et al., 2001).
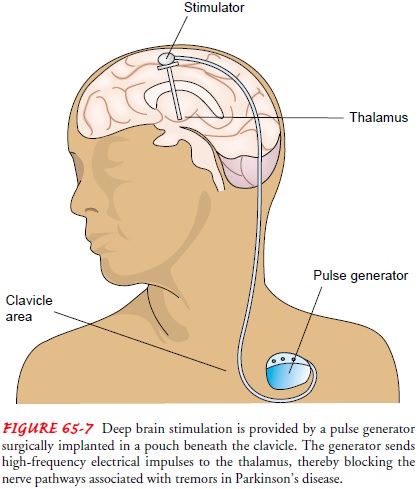
In deep brain stimulation, an electrode is placed in the thalamus and connected
to a pulse generator implanted in a subcutaneous subclavicular or abdominal
pouch. The battery-powered pulse generator sends high-frequency electrical
impulses through a wire placed under the skin to a lead anchored to the skull
(Fig. 65-7). The electrode blocks nerve pathways in the brain that cause
tremors. These devices are not without compli-cations, both from the surgical
procedure needed for implanta-tion and from complications (such as lead
leakage) of the device itself (Koller et al., 2001; Obeso et al., 2001).
Related Topics