Chapter: 11th 12th standard bio zoology Human Body higher secondary school
Ozone layer depletion : Ozone hole, Preventing And Effect of Ozone depletions
Carbon sequestration :-
The simple technique is to preserve trees and plants more. Trees, take up carbon-dioxide, break it down in photosynthesis, and store carbon in new wood. It need massive reforestation. Carbon-dioxide can also be sequestrated directly into deep ocean water or into oil wells or some aquifer form which it cannot escape.
Usage of alternate fuels such as nuclear energy, solar power, wind power and hydrogen fuel cells which emit no greenhouse gases are being considered.
Ozone layer depletion
Ozone is a form of oxygen (O3). In the stratosphere (ozonosphere), ozone blocks out the sun's ultraviolet rays and is a lifesaver.
Ozone as a natural sun block
The electromagnetic radiation emitted from the sun includes ultraviolet radiation, which is potentially harmful to most living things since it can damage DNA. The ozone layer screens out the sun's harmful ultraviolet radiation. Even 1% reduction in the amount of ozone in the upper stratosphere causes a measurable increase in the ultraviolet radiation that reaches the earth surface. If there was no ozone at all, the amount of ultraviolet radiation reaching us would be catastrophically high. All living things would suffer radiation burns, unless they were underground, or in the sea.
In the stratosphere, small amount of ozone are constantly being made by the action of sunlight on oxygen. At the sametime, ozone is being broken down by natural processes. The total amount of ozone usually stays constant because its formation and destruction occur at about the same rate. But unfortunately human activity has recently changed that natural balance. Some manufactured substances such as chloroflurocarbons and hydrochloroflurocarbons can destroy stratosphere ozone much faster than it is formed.
Ozone hole:
Ozone loss was first detected in the stratosphere over the Antarctic. The part of the atmosphere where ozone is most depleted is referred as ' Ozone hole' but it is not a real hole just a vast region of the upper atmosphere where there is less ozone than elsewhere.
Ozone-poor air can spread out from the Polar regions and move above other areas. In addition, direct ozone depleted are is also slowly increasing.
Reasons for the Antarctic Ozone hole:
Scientific observations prove that the ozone hole formed over Antarctic is due to compounds of chlorine and bromine formed in the atmosphere. Nearly all of the chlorine and half of the bromine in the stratosphere comes from human activities, the chlorofluocarbons released due to human activities transported up into the upper stratosphere.
The most common Ozone depleting substances (ODS) are chloroflurocarbons (CFC) or freon gases, bromine compounds on halons, nitrogen oxides and methyl bromide. These compounds are liberally released from air-conditioners, freezers, foam insulations, aerosol products, industrial solevents, fire extinguishers and pesticides.
Effect of Ozone depletions:
If the ozone is depleted more ultraviolet radiations (especially ultraviolet B (UVB) will reach the earths surface.
Effect on plants:- will affect crop yield and forest productivity.
Effect on animals:- will cause damage to fish larvae and other small animals
Effect on human health:- Results in non-melanoma skin cancer and melanoma, acute erythem a (sun burn), ocular abnormalities, cataract, affect immune responses.
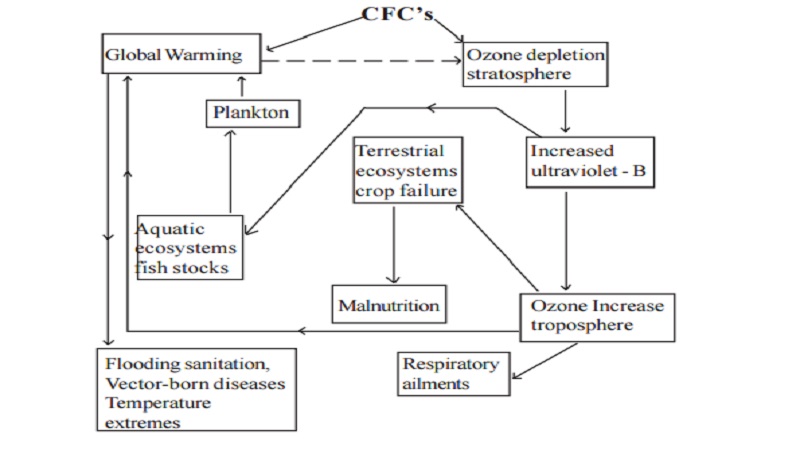
The general effect of ozone depletion is summed up in the following chart
Preventing ozone depletion:
1.CFC's (Chloro Fluro Carbons) should be replaced by HCFC's (Hydro Chloro Fluro Carbons). (If over used could damage ozone), HFC's (Hydro Flouro Carbons), Hydrocarbons such as butane and propane. (but flam-mable and poisonous), Ammonia (must be handled carefully), Water and steam.
2. Production, use and emission of ozone - depleting chemicals should be controlled.
3.Recycling of these chemicals should be increased.
4.Servicing of refrigerators and air-conditioners should be regulated.
5.Refrigirants should be recaptured and used.
Adopt protection measures from sun's radiation.
Related Topics