Chapter: Medical Surgical Nursing: Shock and Multisystem Failure
Overall Management Strategies in Shock
Overall Management Strategies in Shock
As described previously and in the discussion of types of shock to
follow, management in all types and all phases of shock includes the following:
• Fluid replacement to restore
intravascular volume
• Vasoactive medications to
restore vasomotor tone and im-prove cardiac function
• Nutritional support to
address the metabolic requirements that are often dramatically increased in
shock
Therapies described in this section require collaboration among all
members of the health care team to ensure that the manifesta-tions of shock are
quickly identified and that adequate and timely treatment is instituted to
achieve the best outcome possible.
FLUID REPLACEMENT
Fluid replacement is administered in all types of shock. The type of
fluids administered and the speed of delivery vary, but fluids are given to
improve cardiac and tissue oxygenation, which in part depends on flow. The
fluids administered may include crys-talloids
(electrolyte solutions that move freely between intravascu-lar and
interstitial spaces), colloids
(large-molecule intravenous solutions), or blood components.
Crystalloid and Colloid Solutions
The best fluid to treat
shock remains controversial. In emergen-cies, the “best” fluid is often the
fluid that is readily available. Both crystalloids and colloids, as described
later, can be given to restore intravascular volume. Blood component therapy is
used most frequently in hypovolemic shock.
Crystalloids are
electrolyte solutions that move freely between the intravascular compartment
and the interstitial spaces. Isotonic crystalloid solutions are often selected
because they contain the same concentration of electrolytes as the
extracellular fluid and therefore can be given without altering the
concentrations of elec-trolytes in the plasma.
Common intravenous fluids used for resuscitation in hypovo-lemic shock
include 0.9% sodium chloride solution (normal saline) and lactated Ringer’s
solution (Choi et al., 1999). Ringer’s lactate is an electrolyte solution
containing the lactate ion, which should not be confused with lactic acid. The
lactate ion is con-verted to bicarbonate, which helps to buffer the overall
acidosis that occurs in shock.
A disadvantage of using isotonic crystalloid solutions is that three
parts of the volume are lost to the interstitial compartment for every one part
that remains in the intravascular compartment. This occurs in response to
mechanisms that store extracellular body fluid. Diffusion of crystalloids into
the interstitial space ne-cessitates that more fluid be administered than the
amount lost (Choi et al., 1999).
Care must be taken when rapidly administering isotonic crys-talloids to
avoid causing excessive edema, particularly pulmonary edema. For this reason, and
depending on the cause of the hypo-volemia, a hypertonic crystalloid solution,
such as 3% sodium chloride, is sometimes administered in hypovolemic shock.
Hypertonic solutions produce a large osmotic force that pulls fluid from the
intracellular space to the extracellular space to achieve a fluid balance (Choi
et al., 1999; Fein & Calalang-Colucci, 2000). The osmotic effect of
hypertonic solutions re-sults in fewer fluids being administered to restore
intravascular volume. Complications associated with use of hypertonic saline
solution include excessive serum osmolality, hypernatremia, hy-pokalemia, and
altered thermoregulation.
Generally, intravenous colloidal solutions are considered to be plasma
proteins, which are molecules that are too large to pass through capillary
membranes. Colloids expand intravascular vol-ume by exerting oncotic pressure,
thereby pulling fluid into the intravascular space. Colloidal solutions have
the same effect as hy-pertonic solutions in increasing intravascular volume,
but less volume of fluid is required than with crystalloids. Additionally,
col-loids have a longer duration of action than crystalloids because the
molecules remain within the intravascular compartment longer.
An albumin solution is
commonly used to treat hypovolemic shock. Albumin is a plasma protein; an
albumin solution is pre-pared from human plasma and is heated to reduce its
potential to transmit disease. The disadvantages of albumin are its high cost
and limited availability, which depends on blood donors. Syn-thetic colloid
preparations, such as hetastarch and dextran solu-tion, are now widely used.
Dextran, however, may interfere with platelet aggregation and therefore is not
indicated if hemorrhage is the cause of the hypovolemic shock or if the patient
has a co-agulation disorder (coagulopathy).
Complications of Fluid Administration
Close monitoring of the
patient during fluid replacement is neces-sary to identify side effects and
complications. The most common and serious side effects of fluid replacement
are cardiovascular over-load and pulmonary edema.
Patients receiving fluid
replacement must be monitored fre-quently for adequate urinary output, changes
in mental status, skin perfusion, and changes in vital signs. Lung sounds are
aus-cultated frequently to detect signs of fluid accumulation. Adven-titious
lung sounds, such as crackles, may indicate pulmonary edema.
Often a right atrial pressure line (also known as a central ve-nous
pressure line) is inserted. In addition to physical assessment, the right
atrial pressure value helps in monitoring the patient’s re-sponse to fluid
replacement. A normal right atrial pressure value is 4 to 12 mm Hg or cm H2O. Several readings are obtained to determine
a range, and fluid replacement is continued to achieve a pressure within normal
limits. Hemodynamic monitoring with arterial and pulmonary artery lines may be
implemented to allow close monitoring of the patient’s perfusion and cardiac
status as well as response to therapy.
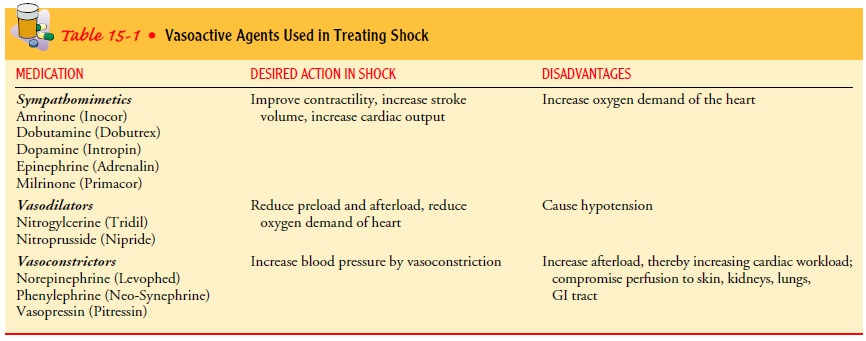
VASOACTIVE MEDICATION THERAPY
Vasoactive medications are administered in all forms of shock to improve
the patient’s hemodynamic stability when fluid therapy alone cannot maintain
adequate MAP. Specific medications are selected to correct the particular
hemodynamic alteration that is impeding cardiac output. Specific vasoactive
medications are pre-scribed for the patient in shock because they can support
the pa-tient’s hemodynamic status. These medications help to increase the
strength of myocardial contractility, regulate the heart rate, reduce
myocardial resistance, and initiate vasoconstriction.
Vasoactive medications are selected for their action on recep-tors of
the sympathetic nervous system. These receptors are known as alpha-adrenergic
and beta-adrenergic receptors. Beta-adrenergic receptors are further classified
as beta1-
and beta2-adrenergic
recep-tors. When alpha-adrenergic receptors are stimulated, blood vessels
constrict in the cardiorespiratory and gastrointestinal systems, skin, and
kidneys. When beta1-adrenergic receptors are stimulated, heart rate and myocardial
contraction increase. Whenbeta2-adrenergic receptors are stimulated, vasodilation occurs in the heart
and skeletal muscles, and the bronchioles relax. The medications used in
treating shock consist of various combina-tions of vasoactive medications to
maximize tissue perfusion by stimulating or blocking the alpha- and
beta-adrenergic receptors.
When vasoactive
medications are administered, vital signs must be monitored frequently (at
least every 15 minutes until sta-ble, or more often if indicated). Vasoactive
medications should be administered through a central venous line because
infiltration and extravasation of some vasoactive medications can cause tis-sue
necrosis and sloughing. An intravenous pump or controller should be used to
ensure that the medications are delivered safely and accurately.
Individual medication
dosages are usually titrated by the nurse, who adjusts the intravenous drip
rates based on the physi-cian’s prescription and the patient’s response. Dosages
are changed to maintain the MAP (usually above 80 mm Hg) at a physiologic level
that ensures adequate tissue perfusion.
Dosages of vasoactive
medications should be tapered and the patient should be weaned from the
medication with frequent monitoring (every 15 minutes) of blood pressure. Table
15-1 presents some of the commonly prescribed vasoactive medica-tions used in
treating shock.
NUTRITIONAL SUPPORT
Nutritional support is an important aspect of care for the patient with
shock. Increased metabolic rates during shock increase en-ergy requirements and
therefore caloric requirements. The pa-tient in shock requires more than 3,000
calories daily.
The release of catecholamines early in the shock continuum causes glycogen stores to be depleted in about 8 to 10 hours. Nu-tritional energy requirements are then met by breaking down lean body mass. In this catabolic process, skeletal muscle mass is bro-ken down even when the patient has large stores of fat or adipose tissue.
Loss of skeletal muscle can greatly prolong the recovery time for the patient in
shock. Parenteral or enteral nutritional sup-port should be initiated as soon
as possible, with some form of en-teral nutrition always being administered.
The integrity of the gastrointestinal system depends on direct exposure to
nutrients. Additionally, glutamine (an essential amino acid during stress) is
important in the immunologic function of the gastrointestinal tract, providing
a fuel source for lymphocytes and macrophages. Glutamine can be administered
through enteral nutrition (Rauen & Munro, 1998).
Stress ulcers occur
frequently in acutely ill patients because of the compromised blood supply to
the gastrointestinal tract. There-fore, antacids, histamine-2 blockers (eg, famotidine
[Pepcid], ran-itidine [Zantac]), and antipeptic agents (eg, sucralfate
[Carafate]) are prescribed to prevent ulcer formation by inhibiting gastric
acid secretion or increasing gastric pH.
Related Topics