Chapter: Biology: Physiological Activities in Plants Nutrition
Osmosis: Two types, Condition, Experiment, Absorption
OSMOSIS
If a raisin is kept in a cup of water for some times it will be shown that the raisin will absorb water and become full and flashed. The outer covering of the raisin is a semi permeable membrane. Out side this membrane there is water in the cup, and inside the membrane there is sweet juice of raisin. The amount of water in the cup is more (actually whole amount is water) but that inside the raisin is very little. In this condition water from the cup enter into the concentrated region inside through the semi permeable membrane of raisin. As a result the raisin swollen and filled with juice. It is happened by a special process known as Osmosis. Osimosis may be defined as -a process by which solvent (water) diffuse from an area of high concentration through a semi permeable membrane to a area of low concentration solution is termed as Osmosis. The process continues till the concentration of two solutions become same. Osmosis is a special type of diffusion.
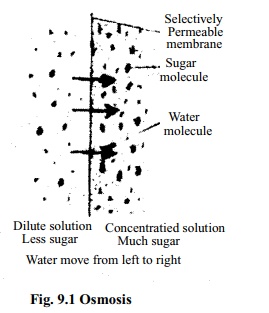
We know solution is a mixture of solute and solvent, for example mixture of sugar and water is a solution. Here water is the solvent and sugar is the solute and the mixture is the solution. In osmosis diffusion of solute never takes place, only solvent diffuses that is in case of plants only water diffuses.
Two types of Osmosis:
1. Endosmosis: osmosis in which solvent (water) from outside enter inside thecell, is called endosmosis. Concentration of cell sap is more than the concentration of the outer solution. Put some raisins in water Water will enter inside the fruits (raisin) and the raisin will swell up like a grape being filled with juice.
2. Exosmosis: Osmosis in which water (solvent) comes out from inside the cellis called exosmosis. Exosmosis takes place when the concentration of external solution is more than the concentration of cell sap.
Put some grape in salt solution and observe it. Water will come out from the grape and it will contract.
Condition of Osmosis:
The process of smosis may occur under the following conditions:
· There should have two solutions. One concentrated and other dilute.
· A semi permeable membrane should separate the two solutions of different concentration.
· The two solutions must be of same solvent.
· Temperature and atmospheric pressure should be same.
Osmotic Pressure: = OP:
In the process of osmosis pressure required from higher concentration area to stop the movement of solvent from lower concentrated solution to higher concentrated solution when a semi permeable membrane separates the
two solutiions, is called Osmotic Pressure (OP). More the difference of concentration of two solutions more the OP, and less the difference of concentration, less will be the OP. if the concentration of two solutions is same there will have no OP and osmosis will not run.
Turgor Pressure
Due to endosmosis water enter into the cell and the cell become swollen. This swollen condition of the cell is called turgidity. Due to turgidity the protoplasm exert a pressure on the cell wall; this pressure is called Turgor Pressure.
An Experiment of Osmosis:
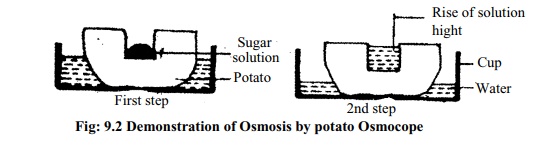
Osmosis can be observed by putting a Potato Osmoscope.
Requirements: A large Potato, A Bowl, Water and Sugar solution.
Procedure: Take half bowl of water. Cut two ends of the potato and make ahole at one end. Now fill up half of the hole of the potato by sugar solution and keep the potato (Potato Osmoscope) in the bowl's water. Care should be taken so that water level should be below the top of the potato cup.
Observation: After some times it will be seen that the level of the solution inthe potato cup is increased.
Conclusion: Outside surrounding the potato there is water and inside the holethere was sugar solution. Cell walls of potato is permeable and the cell membrane is semi permeable (selectively permeable). So the water from the bowl enters through the cell wall and cell membrane to the solution in the hole of the potato. As water enters to high concentration solution through semipermeable membrane so other process is osmosis.
Necessity of Osmosis in Plant Life:
Necessity of osmosis in plant life is unlimited. A short description about this is given bellow:
· Absorption of water: Plant absorbs water by root hairs in the process ofosmosis.
· Movement of water: Water moves inside the, plant body by cell-to-cell ofosmosis.
· Opening and closing of stomata: Opening and closing of stomata iscontrolled by osmosis. So osmosis also controls the rate of transpiration.
· Swelling and growth of cell: For normal growth and expansion of the cell itneeds to swell. Cells become expanded by absorbing water through osmosis. This osmosis plays an important role for the growth of the cell and maintains its normal shape and size.
· Equal distribution of water: Water is essential for almost all the organicactivities. These activities are going on in the living cells. Osmosis is very active to reach water to every living cell from root to leaf.
· Turgidity of the cell: Soft cells become turgid by absorbing water. Cellabsorbs water by osmosis.
· Germination of seeds: The success of germination depends on the process ofosmosis.
Absorption of water & minerals by the plant :
Both water and minerals are essential for plants. Plants absorb water and minerals from soil. The process of absorption of water and minerals is described very briefly :
Absorption of water:
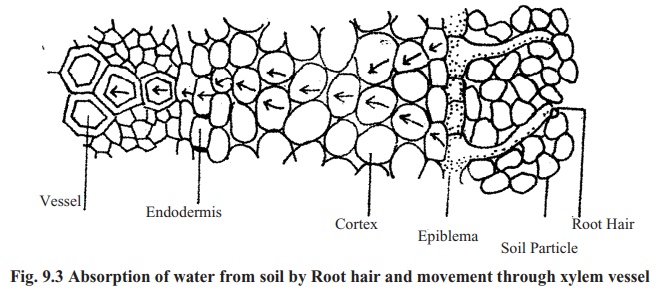
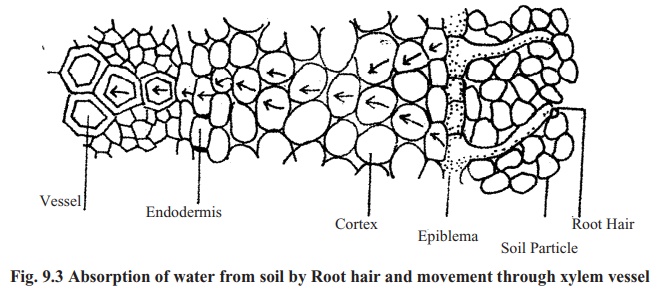
The higher plants absorb water from soil by their root hairs. Root hair region is termed as absorption region. Unicellular root hairs are specially modified epidermal cell for absorption of water. Cell walls of root hairs are made of cellulose and thus it is permeable. Beneath the cell wall, there is the cell membrane, which is selectively permeable. Inside the root hair the cell sap is concentrated and in the outside there is water in between soil particles. So water from soil enter into the cell through permeable cell wall and selectivelypermeable cell membrane by osmosis. Afterwards this water from root hair reaches the vessels of xylem tissue by cell-to-cell osmosis (shown in fig; 9.3). From vessels the water moves to leaves.
Absorption of minerals :
Cell division region at the top of the root is known as mineral absorbing area, some salts also absorb by root hair. Plant cannot take solid salts. The salts (minerals) dissolve in soil-water and converted to Cation (+) and Anion (-), root absorb them as ions. Na+ is a Cation Cl_ is an Anion. K, Ca are Cations; NO3_, PO4_, SO_4 are Anions.
Difference between Absorption of Water and Minerals:
Water absorbed directly and minerals are taken as ions. Water absorbed by root hairs and minerals are taken by cell division region at the top of the root. Water is absorbed by osmosis and some portion of minerals is taken by diffusion.
Related Topics