Chapter: 11th 12th std standard Geography earth space Higher secondary school College Notes
Nutrient cycles and ecosystems
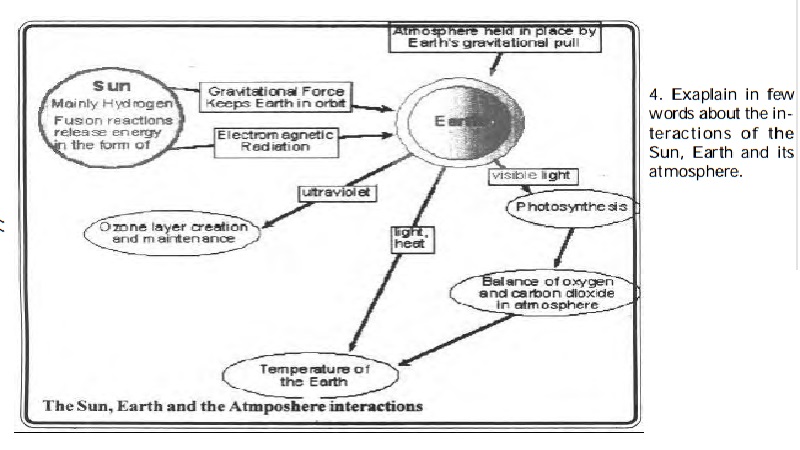
NUTRIENT CYCLES AND ECOSYSTEMS
Our
planet is the only place in the universe that supports life. Life on Earth
requires a variety of organic and inorganic nutrients. These nutrients
continuously recycle through the interactions of organisms and their
environments. Recycling chemicals essential to life involves both geological
and biological processes. These pathways are called biogeochemical cycles and
have three things in common.
1. Reservoirs : These are where the
chemical is held in large quantities for long periods of time.
2. Exchange pools : This is where the
chemical is held for only a short time.
3. Residence time : It is the length of
time a chemical is held in an exchange pool or a reservoir.
For example, the oceans are a
reservoir for water cycle, while a cloud is an exchange pool. Water may reside
in an ocean for thousands of years, but in a cloud for a few days at best. The
biotic community also serves as an exchange pool and also move chemicals from
one stage of the cycle to another. For instance, the trees of the tropical rain
forest bring water up from the forest floor to be evaporated into the atmosphere.
Likewise, coral polyps take carbon from the water and turn it into limestone
rock. The energy for most of the transportation of chemicals from one place to
another is provided either by the sun or by the heat released from the mantle
and core of the Earth.
The
Carbon Cycle
Respiration takes carbohydrates and
oxygen, combines them to produce carbon dioxide, water, and energy.
Photosynthesis takes carbon dioxide and water, produces carbohydrates and
oxygen. The outputs of respiration are the inputs of photosynthesis, and the
outputs of photosynthesis are the inputs of respiration. The reactions are also
complementary in the way they deal with energy. Photosynthesis takes energy
from the sun and stores it as carbohydrates; respiration releases that energy.
Both plants and animals carry on respiration, but only plants can carry on photosynthesis.
The Nitrogen Cycle
The nitrogen cycle is one of the most difficult of the
cycles to learn, simply because there are so many important forms of nitrogen,
and because organisms are responsible for each of the interconversions.
Nitrogen is critically important in forming the amino portions of the amino
acids which in turn form the proteins of our body. Proteins make up skin and
muscle, among other important structural portions of our body, and all enzymes
are proteins. Since enzymes carry out almost all of the chemical reactions in
our body, it's easy to see how important nitrogen is.
The Phosphorous Cycle :
The phosphorous is the simplest of all cycles.
Phosphorous has only one form, phosphate, which is a phosphorous atom with 4
oxygen atoms. This molecule never makes its way into the atmosphere, it is
always part of an organism, dissolved in water, or in the form of rock. When
rock with phosphate is exposed to water especially water with a little acid in
it, the rock is weathered out and goes into solution. Plants take this
phosphorous up through their roots and use it in a variety of ways.
Related Topics